Abstract
Mitomycin-C is used widely in the treatment of malignancies and is associated with serious dose related adverse effects including the occurrence of hemolytic uremic syndrome. We report a patient with a colonic adenocarcinoma who developed hemolytic uremic syndrome after receiving 85 mg/m2 of the drug. The patient was left untreated and died 5 months after this diagnosis was made, of a second malignancy. Differential diagnosis and treatment of this condition are discussed.
INTRODUCTION
Mitomycin-C (MMC) is an alkylating agent, used widely in the treatment of a variety of malignancies. Like other antineoplastic agents, MMC is associated with serious adverse effects with bone marrow suppression being the most common followed by hemolytic uremic syndrome (HUS). There are also rare reports of pulmonary fibrosis and veno-occlusive disease attributed to MMC.Citation[[1]] Although this drug is extensively used, there are no reports of MMC induced HUS from this country. The main emphasis of this case report is to create awareness of this devastating complication and to facilitate the careful use of this drug.
CASE REPORT
A 53 years old female presented with pain in abdomen, loss of appetite and weight loss of one-month duration at a district hospital. She was a known hypertensive and diabetic for 9 years, which were controlled on amlodipine and glibenclamide, respectively. Colonoscopy disclosed a stricture in the ascending colon biopsy from which confirmed an adenocarcinoma. She underwent right hemicolectomy and histopathology confirmed the presence of the adenocarcinoma invading up to the submucosa. Following surgery, she was administered six cycles of intra-venous chemotherapy consisting of MMC 20 mg (cumulative dose of 85 mg/m2), 5 fluorouracil 750 mg and cyclophosphamide 1 gm at four weekly intervals. Prior to starting chemotherapy her blood urea was 30 mg/dL, serum creatinine of 0.9 mg/dL and urine examination did not reveal any abnormality.
A month following completion of chemotherapy, she developed a further decrease in her appetite and progressive pallor. Laboratory examination revealed a hemoglobin level of 6 gm/dL, platelet count of 88 000/mm3 and serum creatinine of 2.9 mg/dL. The renal failure worsened over the next three weeks and serum creatinine increased to 6.1 mg/dL. She was transfused three units of blood and referred to this Institute for evaluation of renal failure. Her general physical examination revealed moderate pallor. Her blood pressure was 170/90 mm of Hg and required amlodipine for its control. Systemic examination did not reveal any abnormality.
Urine examination revealed 1+ albuminuria with a 24-h urine protein excretion of 740 mg. Her hemoglobin was 7.7 gm/dL with a reticulocyte count of 0.2%, total leukocyte count was 6100/mm3 with normal differential counts. Her platelet count was 1.03 lakhs/mm3 at admission, which increased a week later to 1.5 lakhs/mm3 and blood film showed anisocytosis with microcytes. Renal function tests revealed a blood urea of 98 mg/dL and serum creatinine of 6.1 mg/dL. Blood sugar and liver function tests were within normal limits and the serum lactate dehydrogenase level was 571 U/L (normal 313–618 U/L). Abdominal ultrasonography and CT Scan did not reveal any evidence of recurrence of tumor and kidney sizes were normal with compact pelvicalyceal systems.
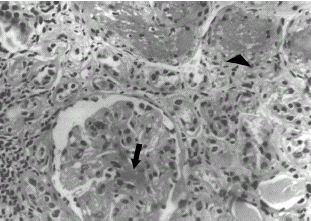
Percutaneous renal biopsy was done to ascertain the cause of renal failure and revealed fifteen glomeruli all of which were shrunken and bloodless with obliteration of capillaries, with some glomeruli also showing fibrin thrombi. The glomerular basement membrane showed irregular thickening at places and there was onion peel appearance of arterioles with fibrinoid necrosis (), confirming the diagnosis of HUS. On indirect immunofluorescence there were fibrin deposition in the arterioles and significant Ig M and C3 deposits in the mesangium suggesting an immune complex nature of the disease. Since there was no evidence of ongoing microangiopathic hemolysis and platelet counts had returned to normal, specific therapy for HUS was not considered and patient was discharged on conservative therapy for chronic renal failure. Her serum creatinine continued to remain between 5–6 mg/dL on follow up. Five months after she was discharged, she died of a second malignancy (ovarian cancer) and until pre-terminally, she did not require dialytic support.
DISCUSSION
Nephrotoxicity of MMC is well documented in the literature and approximately 10% of patients treated with MMC develop HUS. Lesesne et al.Citation[[2]] reviewed the United States National Registry data of cancer associated HUS. Amongst 85 patients with cancer associated HUS, 84 patients had received MMC part of their anti-neoplastic chemotherapy regimen and 89% of all patients with HUS had an adenocarcinoma. MMC associated HUS is dose dependent. In the US Registry data, all but nine patients received a cumulative dose of MMC greater than 60 mg/m2 and only 2.5% of patients receiving cumulative dose of MMC ≤ 50 mg/m2 developed HUS. The incidence of HUS increases to 28% with cumulative doses exceeding 70 mg/m2.Citation[[1]] Our patient received a cumulative dose of 85 mg/m2, much more than the dose that predisposes to the development of HUS. In more than 75% of cases, HUS develops within 4 months of the last dose of MMC. Mean time for the development of HUS from last MMC dose was reported to be 92 days with a range of 1 week to 15 months.Citation[[2]] In a prospective study to predict development of MMC induced HUS, only one amongst 37 study subjects developed the disease and none of the laboratory parameters predicted the development of HUS. In patients not developing HUS, all laboratory parameters to detect early evidence of hemolysis remained normal.Citation[[3]]
In patients with disseminated malignancies, a syndrome resembling HUS can be mimicked with the development of disseminated intravascular coagulation (DIC), however the characteristic coagulation abnormalities associated with DIC will differentiate it from HUS. Further, disseminated malignancies can also cause a microangiopathic hemolytic anemia secondary to damage to the red blood cells within the microvasculature invaded by the tumor and absence of thrombocytopenia and renal failure will usually differentiate this condition from HUS.Citation[[2]] Rarely, in patients with a malignancy, HUS can also develop secondary to drugs like bleomycin or cisplatin.Citation[[5]]
Clinical manifestations include occurrence of rapidly progressive renal failure, hypertension, pulmonary edema and neurological dysfunction. In addition to renal failure the laboratory investigations reveal evidence of microangiopathic hemolytic anemia and thrombocytopenia, which may be transient. Adverse reactions to blood transfusions are frequent and at times can be life threatening. These are reported in 65% of patients who receive blood transfusions, usually in the form of non-cardiogenic pulmonary edema, worsening of microangiopathy and neurological deterioration.Citation[[2]] MMC induced HUS is associated with high mortality, either as a direct result of HUS or due to progressive cancer.
Postulated mechanisms of renal damage include endothelial damage either directly attributable to the toxic effects of the drug or indirectly by immune complex formation. Immune complexes may cause endothelial damage by platelet activation and aggregation.Citation[[4]] Circulating immune complexes can also be detected frequently in these patients. Treatment modalities that have been tried include corticosteroids, antiplatelet agents, heparin, plasma exchange and staphylococcal protein A (SPA) immunopheresis. Amongst these, only plasma exchange and SPA immunopheresis have been found to be beneficial. Mechanism of improvement is through depletion of immune complexes and levels of circulating immune complexes govern duration of therapy. Efficacy of SPA immunopheresis was confirmed in a multicenter study that included 55 patients (43 of whom had renal failure) of whom 25 responded to treatment. Clinical response correlated with disappearance of circulating immune complexes. Renal function only stabilized in responders, however no improvement in renal function was noted after this treatment.Citation[[5]] Beneficial effect of plasmapheresis has only been described in a few case reports.Citation[[6]], Citation[[7]]
We conclude that MMC induced HUS is a dose related side effect, which can be prevented by not exceeding a cumulative dose of 60 mg/m2. Although SPA immunopheresis and possibly plasmapheresis halts the microangiopathic process, improvement in renal function with treatment is not expected. Development of HUS following MMC is associated with a high mortality rate.
REFERENCES
- Chabner B.A., Myers C.E. Clinical Pharmacology of Cancer Chemotherapy. Cancer: Principles and Practice of Oncology, 3rd Ed., V.T. De Vita, S. Hellman, S.A. Rosenberg. JB Lippincott Company, Philadelphia 1989; 349–395
- Lesesne J.B., Rothschild N., Erickson B., Korec S., Sisk R., Kellar J., Arbus M., Woolley P.V., Chiazze L., Schein P.S., Messerschmidt G.L. Cancer Associated Hemolytic Uremic Syndrome: Analysis of 85 Cases from a National Registry. J. Clin. Oncol. 1989; 7: 781–789
- Verwey J., Vries J.D., Pinedo H.M. Mitomycin C Induced Renal Toxicity, A Dose Dependent Side Effect?. Eur. J. Cancer Clin. Oncol. 1987; 23: 195–198
- Jain S., Seymour A.E. Mitomycin C Associated Hemolytic Uremic Syndrome. Pathology 1987; 19: 58–61
- Snyder H.W., Mittelman A., Oral A., Henry D.H., Korec S., Bertram J.H., Guthrie T.H., Jr., Ciavarella D., Wuest D. Treatment of Cancer Chemotherapy-Associated Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome by Protein A Immunoadsorption of Plasma. Cancer 1993; 71: 1882–1892
- Garibotto G., Acquarone N., Saffioti S., Deferrari G., Villagio B., Ferrario F. Successful Treatment of Mitomycin C Associated Hemolytic Uremic Syndrome by Plasmapheresis. Nephron 1989; 51: 409–412
- Chow S., Roscoe J., Cattran D.C. Plasmapheresis and Antiplatelet Agents in the Treatment of the Hemolytic Uremic Syndrome Secondary to Mitomycin. Am. J. Kid. Disease 1986; 7: 407–412