Abstract
Glomerular mesangial cells play a major role in glomerular hemodynamics, considered also as antigen-presenting cells participating in immune response. Mesangial dysfunction and proliferation are typical lesions of diabetic glomerulopathy. Adenosine, a local hormone, produced by mesangial cells is a metabolic regulator of renal blood flow, capable of decreasing glomerular filtration rate (GFR), exerting immunosuppressive, antiproliferative and anti-inflammatory properties. Since it was well established that antioxidants confer protection against increased oxidative stress that occurs in diabetes, the effect of captopril, reduced glutathione and melatonin on adenosine metabolism was investigated. Glomerular mesangial cells obtained from collagenase treated glomeruli, isolated from renal cortex of Sprague-Dowley rats, were grown under high glucose conditions (30 mmol/L) as a model of diabetic microenvironment. The activity of adenosine metabolizing enzymes: 5′-nucleotidease (5′-NU) responsible for its production and adenosine deaminase (ADA)—responsible for its degradation were investigated. Hyperglycemic conditions led to decreased adenosine production via 5′-NU and decreased removal via ADA. Captopril, given in therapeutic concentration induced enzyme activities in normoglycemic conditions and restored hyperglycemia—induced decrease. In order to investigate if the presence of SH groups may be responsible for this improvement, the cells were exposed to reduced glutathione, and it exerted almost equal effect, given in physiological and higher concentrations. Melatonin increased 5′-NU activity only in physiological glucose conditions. Presented results confirm potential renoprotective effect of SH-group containing antioxidant supplementation during diabetes in restoring adenosine metabolism.
INTRODUCTION
Glomerular mesangial cells play a major role in glomerular hemodynamics. They are considered also as antigen-presenting cells, participating in immune and inflammatory response to glomerular injury. The interaction of mesangial cells with different components of extracellular matrix proteins can have an influence on their phenotype, associated with the changes in their attachment, contraction, migration, survival and proliferation. The disturbance in their functional activities represents an important mechanism in pathogenesis of progressive glomerulopathy in diabetic humans and animal models.Citation[[1]], Citation[[2]]
Adenosine has been implicated in the decrease of glomerular filtration rate (GFR), because of its vasoactive properties, via contraction of afferent arteriole. Rat glomeruli have been shown to exhibit functional coexistence of both, A1 and A2 receptors, included in opposite regulation of c-AMP concentration,Citation[[3]], Citation[[4]] while adenosine-induced mesangial cell contraction occurs by its binding to A1 receptors.Citation[[5]] It is also included in tubulo-glomerular feedback mechanisms, by inhibiting renin release, independent of its hemodynamic property.Citation[[6]] Adenosine exerts potent immunosuppressive and anti-inflammatory properties; it also inhibits reactive oxygen radical metabolite production from mesangial cells.Citation[[1]], Citation[[7]], Citation[[8]], Citation[[9]] Adenosine-induced cell apoptosis may be important in limiting cell proliferation rate.Citation[[10]] Adenosine therapy may improve the pathophysiology of tissue ischemic failure by increasing ischemic preconditioning.Citation[[11]] Since adenosine turnover primarily depends on its synthesis via enzyme 5′-nucleotidase and degradation via enzyme adenosine deaminase, the alteration in their activity would result in the overall adenosine concentration.Citation[[12]]
This study tried to evaluate the mechanism of the changes in mesangial cells adenosine metabolism, which may have relevance to the early glomerular hemodynamic changes observed in diabetic nephropathy. Glomerular mesangial cells cultured under high glucose conditions were employed as a model of diabetic microenvironment. At the same time the study was aimed to evaluate the effect of different pluripotent antioxidants (captopril, reduced glutathione-GSH, and melatonin) on adenosine metabolism.
MATERIALS AND METHODS
Glomerular mesangial cells were obtained from collagenase treated glomeruli isolated from the renal cortex of Sprague-Dawley rats, described by Foidart et al.Citation[[13]] Glomeruli were isolated by sieving techniques and centrifugation, afterwards they were cultured in RPMI-1640 medium, supplemented with 10% FCS, buffered with HEPES 20 mmol pH 7.2, 50 U/mL penicillin and 50 µg/mL streptomycin sulphate. After three weeks of primary culture, mesangial cells were dissociated using 0.05% trypsin in 0.02% EDTA, passed through a 50 µm sieve and transferred to plastic Petri dishes. When ricked a confluence, the cells were detached and seeded in 24-well plates. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2–95% air. After two passages the cells were divided in different experimental groups: I control (cultured under physiological 5 mmol glucose concentration); II hyperglycemia (cultured under 30 mmol glucose concentration); III, IV, and V groups were for the evaluation of dose dependent effects of captopril (2 × 10−5, 10−4, and 2 × 10−4 mol), cultured under normal glucose conditions; VI, VII, and VIII were for the evaluation of dose dependent effects of reduced glutathione GSH (5 × 10−3, 10−2, and 2 × 10−2 mol) cultured under normal glucose conditions; IX, X, and XI were for the evaluation of dose dependent effects of melatonin (10−6, 10−5, and 10−4 mol) cultured under normal glucose conditions; XII was cultured with captopril (2 × 10−5 mol) under high glucose conditions; XIII was cultured with GSH (10−2 mol) under high glucose conditions and XIV was cultured with melatonin (10−5 mol) under high glucose conditions. Cells were exposed to glucose and antioxidants for 48 h.
The activity of 5′-nucleotidase was measured according to the Wood and Williams methodCitation[[14]] modified for isolated cells by Kocic et al.,Citation[[15]], Citation[[16]] while the activity of adenosine deaminase was measured according to the Pederson and Berry procedure,Citation[[17]] modified by Lauber.Citation[[18]] Protein concentration was measured according to the Lowry procedure.Citation[[19]]
Enzyme activities are µmoles of product (inorganic phosphate or ammonia) liberated per gram of cell protein and per minute. Means ± standard deviations (SD) are given throughout the text. Statistical significance was currently estimated using Student's t-test.
RESULTS
The results of 5′-nucleotidase (5′-NU) and adenosine deaminase (ADA) activity are shown in and . When glucose was added to the incubation medium at a concentration of 30 mmol, 5′-nucleotidase (5′-NU) activity was significantly inhibited (a). Significant stimulation of the basal activity of 5′-nucleotidase was observed during incubation with captopril at the therapeutic concentration (a), but the concentrations higher than therapeutic ones declined enzyme activity toward control. A marked increase of 5′-NU activity with dose-dependent exposure to reduced glutathione (GSH) and melatonin was observed. (b and c). Among investigated antioxidants only captopril and GSH were capable of counteracting inhibition of 5′-nucleotidase during hyperglycemic conditions (a).
Figure 1. Glomerular mesangial cells were cultured in RPMI-1640 medium, supplemented with 10% FCS, buffered with HEPES 20 mmol pH 7.2, 50 U/mL penicillin and 50 µg/mL streptomycin sulphate in the presence of physiological (5 mmol) and hyperglycemic (30 mmol) conditions. Six groups of cells were exposed to antioxidants: captopril (2 × 10−5 mol), glutathione (GSH 10−2 mol) or melatonin (10−5 mol) and simultaneously with 30 mmol glucose for 48 h. Means ± SD of enzyme activities are shown for the determinations of six samples. Statistical significance was estimated by comparing with the control value.
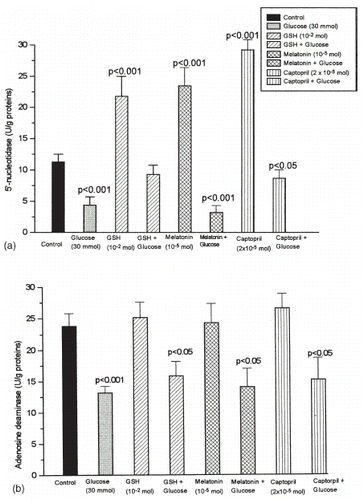
Figure 2. Glomerular mesangial cells were cultured in RPMI-1640 medium, supplemented with 10% FCS, buffered with HEPES 20 mmol pH 7.2, 50 U/mL penicillin and 50 µg/mL streptomycin sulphate. Dose dependent effect of captopril was evaluated by using therapeutic and higher concentrations (2 × 10−5, 10−4, and 2 × 10−4 mol). Dose dependent effects of reduced glutathione GSH was evaluated for physiological and higher concentrations (5 × 10−3, 10−2, and 2 × 10−2 mol). Dose dependent effects of melatonin was evaluated by using therapeutic and higher concentrations (10−6, 10−5, and 10−4 mol) for 48 h. Mean ± SD of enzyme activities are shown for the determination of six ssamples. Statistical significance was estimated by comparing with the control value.
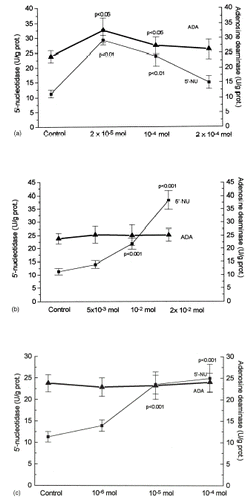
Adenosine deaminase activity of mesangial cells was about two times lower when cells are cultured under hyperglycemic conditions (b). Among investigated antioxidants only captopril, given in therapeutic concentration, was capable of increasing enzyme activity significantly when tested in a dose-dependent manner (a). When antioxidants were given during hyperglycemic conditions they still maintained significant decrease of enzyme activity compared to control conditions.
DISCUSSION
Glomerular hyperfiltration and expansion of mesangial extracellular matrix proteins functionally characterize diabetic nephropathy, presumably by synthesis of type IV collagen and fibronectin. Mesangial cells are the sites of typical proliferative lesions, accompanied with the reduction of contractile responsiveness. Observed functional abnormalities precede the development of microalbuminuria, lead to the end-stage renal failure and requires renal-replacement therapy in diabetes.Citation[[1]] These changes could be attributed to the defective adenosine metabolism. It was recently documented that the elevated level of glucose results in time and concentration-dependent inhibition of adenosine transport.Citation[[20]] As our results suggest, in high glucose conditions the loss of 5′-nucleotidase activity (a) may be responsible for decreased adenosine production and diminished overall adenosine turnover. A bifunctional role of 5′-nucleotidase as membrane ecto-enzyme and the cell receptor for extracellular matrix proteins are well established.Citation[[21]], Citation[[22]] Since adenosine, as an important local hormone with antiproliferative effect, has also been proposed as a metabolic regulator of renal blood flow, capable of increasing mesangial cell contractility, the decrease in contractile function in high glucose conditions may be related to the decrease of 5′-nucleotidase activity. The decrease in 5′-nucleotidase activity is observed also in the presence of higher fibronectin concentrations,Citation[[21]], Citation[[23]] suggesting that the enhanced production of extracellular matrix proteins may be responsible for decreased adenosine production. It is also established that mesangial cell apoptosis represents the major mechanism for resolution of glomerular hypercellularity in mesangial proliferation.Citation[[24]] Adenosine and/or adenosine deaminase deficiency are potent mediators of programmed cell death, through the expression of pro-apoptotic product Bax, the decrease of anti-apoptotic product Bcl-2, increase of cytochrome c release, induction of caspase3-like activity, p53 activation and activation of DNA fragmentation.Citation[[10]], Citation[[25]], Citation[[26]] Thus, decreased adenosine production, that was observed, may counterbalance cell apoptosis thereby mediating increased proliferative potential of mesangial cells. It was demonstrated that hyperglycemia can exert effects through the activation of protein kinase C pathway by an increasing de novo synthesis of diacylglycerol from excessive glucose uptake. The activation of protein kinase C, found also in glomerular mesangial cells cultured under high glucose conditions is responsible for the activation of MAPK cascade which may result in various functional changes, presumably through the activation of cytosolic phospholipase A2.Citation[[27]], Citation[[28]], Citation[[29]], Citation[[30]] Adenosine, acting through A2 receptors, can suppress arachidonic acid release.Citation[[31]] The decrease on adenosine production could provide an explanation for the significant enhancement of arachidonic acid release upon hyperglycemic conditions.
Several lines of evidence point out the importance of renin-angiotensin system in modulation of glomerular filtration and function, by increasing GFR in experimental diabetes. The inhibition of the vasoconstrictor peptide angiotensin II has been shown to delay end-stage renal failure in different types of renal diseases.Citation[[2]], Citation[[32]] The beneficial effect of ACE inhibitors in preventing diabetic nephropathy, associated with the preservation of normal GFR and free-radical scavenger activity, was originally demonstrated.Citation[[1]], Citation[[33]], Citation[[34]], Citation[[35]] Studies aimed to define the factors responsible for altered glomerular hemodynamics in diabetes support the relationship between renin-angiotensin system and adenosine production.Citation[[6]] As obtained results suggest therapeutic concentration of captopril was capable of increasing adenosine production by 5′-nucleotidase and counteracted also the decrease obtained in hyperglycemic conditions (a and a). It probably represents the additional mechanism of the beneficial effect of ACE inhibitors on improvement of glomerular hemodynamics.Citation[[34]] These facts support the proposal that the beneficial effects of ACE-I on the progression of nephropathy may be independent on its antihypertensive properties.
It was well documented that the alteration of redox state may have an influence on mesangial cells chemokine expression, while both prooxidative redox state (presumably due to decreased GSH content) and hyperglycemia may have implications on injury-associated alterations of mesangial matrix generation.Citation[[36]], Citation[[37]], Citation[[38]] Several studies have shown that the decrease in intracellular concentration of reduced glutathione (GSH) in the kidneys increases the sensitivity of these organs to oxidative injury and susceptibility to cellular disruptions, while the addition of antioxidants may provide protection against cellular injury.Citation[[39]] Reduced glutathione (GSH) exerted activatory effect on 5′-nucleotidase activity more than on adenosine deaminase activity, under normal, as well as under hyperglycemic conditions ( and b), suggesting that the antioxidative properties of SH groups of GSH may be responsible for the prevention of oxidative modification of 5′-nucleotidase.Citation[[16]] Since GSH was almost equally able to restore adenosine deaminase activity (b), it means that GSH was able to recover near physiological steady state of adenosine metabolism.
Beside its contribution to circadian a seasonal rhythm, melatonin has been found to have number of other actions, with research centering on the broad-spectrum antioxidative properties.Citation[[40]], Citation[[41]] The direct action of melatonin on the renal system in reducing oxidative stress has been implicated in protecting kidneys against free-radical induced, as well as diabetic nephropathy with considerable potential for therapeutical use.Citation[[42]], Citation[[43]], Citation[[44]], Citation[[45]] In presented study melatonin was able to activate 5′-nucleotidase under physiological glucose conditions, without change of the adenosine deaminase activity, acting by increasing adenosine substrate pool. But it was not able to confer the protection of these enzymes in hyperglycemic conditions. The possible explanation can be hyperglycemia-induced stimulation of protein kinase C, since activators of protein kinase C can prevent and reverse melatonin effects.Citation[[46]]
Functional identification of adenosine metabolism in mesangial cells seems to be a promising step toward new targets for effective drug combination during sustained hyperglycemia. Presented results confirm potential renoprotective effect of SH-group containing antioxidant supplementation during diabetes in restoring adenosine metabolism.
REFERENCES
- Buschaunsen L., Seibold S., Gross O., Matheaus T., Weber M., Schulze-Lohoff E. Regulation of Mesangial Cell Function by Vasodilatory Signaling Molecules. Cardiovasc. Res. 2001; 51(3)463–469
- Parving H.H. Renoprotection in Diabetes: Genetic and Non-Genetic Risk Factors and Treatment. Diabetologia 1998; 41(7)745–759
- Olivera A., Tomas M., Lopez-Novoa J.M. Effect of Adenosine A1 and A2 Agonists and Antagonists on cAMP and Ca2+ in Cultured Rat Mesangial Cells. Am. J. Physiol. 1992; 262(4)C840–C844
- Stefanovic V., Savic V., Vlahovic P., Ardallou N. Ecto 5′-Nucleotidase of Cultured Rat Mesangial Cells. Renal Physiol. Biochem. 1988; 11(1–2)89–100
- Olivera A., Lamas S., Rodriguez-Puyol D., Lopez-Novoa J.M. Adenosine Induces Mesangial Cell Contraction by an A1-Type Receptor. Kidney Int. 1989; 35(6)1300–1305
- Spielman W.S., Thompson C.I. A Proposed Role for Adenosine in the Regulation of Renal Hemodynamics and Renin Release. Am. J. Physiol. 1982; 242(5)F423–F435
- Kammer G.M. Adenosine: Emerging Role as an Immunomodifying Agent. J. Lab. Clin. Med. 1987; 110(3)255–256
- Nosaka K., Takahashi T., Miyanoshita A., Nishi T., Suzuki K., Kurokawa K., Endou H. Effect of Adenosine on Phorbol Myristate Acetate Induced-Reactive Oxygen Metabolite Production in Cultured Mesangial Cells. Free Rad. Biol. Med. 1996; 20(1)151–155
- Schulze-Lohoff E., Ogilvie A., Sterzel R.B. Extracellular Nucleotides as Signaling Molecules for Renal Mesangial Cells. J. Auton. Pharmacol. 1996; 16(6)381–384
- Wakade T.D., Palmer K.C., McCauley R., Przywara D.A., Wakade A.R. Adenosine-Induced Apoptosis in Chick Embryonic Sympathetic Neurons: A New Physiological Role of Adenosine. J. Physiol. 1995; 488(Pt3)123–138
- Kitakaze M., Hori M. It is a Time to Ask What Adenosine Can Do for Cardioprotection. Heart Vessels 1998; 13(5)211–228
- Fox I.H., Kelley W.N. The Role of Adenosine and Deoxyadenosine in Mammalian Cells. Ann. Rev. Biochem. 1978; 47: 655–686
- Foidart J.B., Dechenne C.A., Mahieu P., Creutz C.E., De Mey J. Tissue Culture of Normal Rat Glomeruli. Isolation and Morphological Characterization of Two Homogenous Cell Lines. Invest. Cell. Pathol. 1979; 2(1)15–26
- Wood R.J., Williams D.G. Colorimetric Determination of Serum 5′-Nucleotidase without Deproteinization. Clin. Chem. 1981; 27(3)464–465
- Kocic G., Vlahovic P., Pavlovic D., Kocic R., Jevtovic T., Cvetkovic T., Stojanovic I. The Possible Importance of the Cation-Binding Site for the Oxidative Modification of Liver 5′-Nucleotidase. Arch. Physiol. Biochem. 1998; 106(2)91–99
- Kocic G., Pavlovic D., Jevtovic T., Kocić R., Bojić A., Djordjević V., Sokolović D., Djindjić B. Oxidative Modification of Rat Liver 5′-Nucleotidase: The Mechanisms of Protection and Reactivation. Arch. Physiol. Biochem. 2001; 109(4)323–330
- Pederson R.C., Berry A.J. Sensitive, Optimized Assay for Serum AMP Deaminase. Clin. Chem. 1977; 23(9)1726–1733
- Lauber K. Photometric Determination of Nitrogen. Wet Incineration Followed by Formation of Indophenol Blue with Salicylate/Hypochlorite. Clin. Chim. Acta 1976; 67(1)107–110
- Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951; 193: 265–275
- Montecinos V.P., Aguayo C., Flores C., Wyatt A.W., Pearson J.D., Mann G.E., Sobrevia L. Regulation of Adenosine Transport by d-Glucose in Human Fetal Endothelial Cells: Involvement of Nitric Oxide, Protein Kinase C and Mitogen-Activated Protein Kinase. J. Physiol. 2000; 15(3)777–790
- Olmo N., Turnay J., Risse G., Detzmann R., Von der Mark K., Lizarbe M.A. Modulation of 5′-Nucleotidase Activity in Plasma Membranes and Intact Cells by the Extracellular Matrix Proteins Laminin and Fibronectin. Biochem. J. 1992; 282(1)181–188
- Stochaj U., Richter H., Mannherz H.G. Chicken Gizzard 5′-Nucleotidase is a Receptor for the Extracellular Matrix Component Fibronectin. Eur. J. Cell. Biol. 1990; 51(2)335–338
- Dieckhoff J., Mollenhauer J., Kuhl U., Niggemeyer B., Von der Mark K., Mannherz H.G. The Extracellular Matrix Proteins Laminin and Fibronectin Modify the AMP-ase Activity of 5′-Nucleotidase from Chicken Gizzard Smooth Muscle. FEBS Lett. 1986; 195(1–2)82–86
- Baker A.J., Mooney A., Hughes J., Lombardi D., Johnson R.J., Savill J. Mesangial Cell Apoptosis: The Major Mechanism for Resolution of Glomerular Hypercellularity in Experimental Mesangial Proliferative Nephritis. J. Clin. Invest. 1994; 94(5)2105–2116
- Imura T., Shimohama S. Opposing Effects of Adenosine on the Survival of Glial Cells Exposed to Clinical Ischemia. J. Neurosci. Res. 2000; 62(4)539–546
- Benveniste P., Cohen A. p53 Expression is Required for Thymocyte Apoptosis Induced by Adenosine Deaminase Deficiency. Proc. Natl. Acad. Sci. USA 1995; 92(18)8373–8377
- Williams B., Schrier R.W. Glucose-Induced Protein Kinase C Activity Regulates Arachidonic Acid Release and Eicosanoid Production by Cultured Glomerular Mesangial Cells. J. Clin. Invest. 1993; 92(6)2889–2896
- Ayo S.H., Radnik R., Garoni J.A., Troyer D.A., Kreisberg D.I. High Glucose Increases Diacylglycerol Mass and Activates Protein Kinase C in Mesangial Cell Culture. Am. J. Physiol. 1991; 261(4Pt2)F571–F577
- Haneda M., Araki S., Togawa M., Sugimoto T., Isono M., Kikkawa R. Mitogen-Activated Protein Kinase Cascade is Activated in Glomeruli of Diabetic Rats and Glomerular Mesangial Cells Cultured Under High Glucose Conditions. Diabetes 1997; 46(5)847–853
- Ishii H., Jirousek M.R., Koya D., Takagi C., Xia P., Clermont A., Bursell S.E., Kern T.S., Ballas L.M., Health W.F., Stramm L.E., Feener E.P., King G.L. Amelioration of Vascular Dysfunctions in Diabetic Rats by an Oral PKC Inhibitor. Science 1996; 272(5262)728–731
- Krump E., Borgeat P. Adenosine. An Endogenous Inhibitor of Arachidonic Acid Release and Leukotriene Biosynthesis in Human Neutrophils. Adv. Exp. Med. Biol. 1999; 447: 107–115
- Lewis E., Hunsicker L., Bain R., Rhode R. The Effect of Angiotensin-Converting Enzyme Inhibition on Diabetic Nephropathy. N. Engl. J. Med. 1993; 329(20)1456–1462
- Mathiesen E.R., Hommel E., Hansen H.P., Parving H.H. Preservation of Normal GFR with Long-term Captopril Treatment in Normotensive IDDM Patients with Microalbuminuria. J. Am. Soc. Nephrol. 1997; 8: 115A
- Ichikawi I., Harris R.C. Angiotensin Actions in the Kidney: Renewed Insight into the Old Hormone. Kidney Int. 1991; 40(4)583–596
- Egan T.M., Minta J.O., Scrimgeour K.G., Cooper J.D. Captopril—A Potential Free Radical Scavenger: Inhibition of PMN NADPH Oxidase. Clin. Invest. Med. 1988; 11(5)351–356
- Rovin B.H., Dickerson J.A., Tan L.C., Fassler J. Modulation of IL-1 Induced Chemokine Expression in Human Mesangial Cells Through the Alteration in Redox Status. Cytokine 1997; 9(3)178–186
- Shan Z., Tan D., Satriano D., Silbiger S., Schlondorf D. Intracellular Glutathione Influences Collagen Generation by Mesangial Cells. Kidney Int. 1994; 46(2)388–395
- Chin T.A., Templeton D.M. Protective Elevations of Glutathione and Metallothionein in Cadmium-Expressed Mesangial Cells. Toxicology 1993; 29(1–2)145–156
- Parks L.D., Zalups R.K., Barfuss D.W. Heterogeneity of Glutathione Synthesis and Secretion in the Proximal Tubule of the Rabbit. Am. J. Physiol. 1998; 274(5)F924–F931
- Morgan P.J. Molecular Signaling via the Melatonin Receptor. Adv. Pineal Res. 1991; 5: 191–197
- Vanecek J. Cellular Mechanisms of Melatonin Action. Physiol. Rev. 1998; 78(3)687–721
- Montilla P., Tunez I., Munoz M.C., Lopez A., Soria J.V. Hyperlipidemic Nephropathy Induced by Adriamycin: Effect of Melatonin Administration. Nephron 1997; 76(3)345–350
- Daniels W.M., Reiter R.J., Melchiorri D., Sewerynek E., Pablos M.I., Ortiz G.C. Melatonin Counteracts Lipid Peroxidation Induced by Carbon Tetrachloride but does not Restore Glucose-6 Phosphatase Activity. J. Pineal Res. 1995; 19(1)1–6
- Sener G., Satiroglu H., Kabasakal L., Arbak S., Oner S., Ercan F., Keyer-Uysa M. The Protective Effect of Melatonin on Cisplatin Nephrotoxicity. Fundam. Clin. Pharmacol. 2000; 14(6)553–560
- Ha H., Yu M.R., Kim K.H. Melatonin and Taurine Reduce Early Glomerulopathy in Diabetic Rats. Free Rad. Biol. Med. 1999; 26(7–8)944–950
- Sugden D., Rowe S.J. Protein Kinase C Activation Antagonizes Melatonin-Induced Pigment Activation in Xenopus Laevis Melanophores. J. Cell. Biol. 1992; 119(6)1515–1521
