Abstract
Acute renal failure (ARF) is a serious damage of renal function induced by various nephrotoxic drugs, ischemia, bilateral urethral obstruction, trauma and unilateral nephrectomy. Dramatic clinical syndrome, azotemia, develops as a result of hypovolemia, oliguria, reduced glomerular filtration and acidosis. In addition to classic medications recent studies give more attention to beneficial effect of natural plant products as bioflavonoids. We have studied the influence of bioflavonoid, quercetin, on hepatic urea production in glycerol induced ARF in the rats. Male Sprague Dawley rats were used in the experiment. The value of urea production in the liver was determined by measuring of liver arginase activity, the terminal enzyme of urea cycle. Arginase activity was increased (p<0.01) as well as urea level (p<0.001) 48 h after glycerol administration. Pretreatment by quercetin suppressed the arginase activity in the liver (p<0.05) and plasma levels of urea (p<0.01). So, we have concluded that quercetin may be beneficial in glycerol induced ARF.
Introduction
Glycerol induced kidney failure is a result of toxic and ischemic insult to the kidney.Citation[[1]], Citation[[2]] High blood levels of urea, creatinine and other uremic toxins with hormonal and electrolyte misbalance are the reason for the damage of muscle, liver, brain, and other tissues. We have studied the connection between renal cell dysfunction and hepatic functional ability in producing of urea. Plasma levels of urea and creatinine, as markers for kidney function, and liver arginase activity, the key enzyme in urea cycle, were measured. The rate of urea synthesis is under control by hormones and availability of substrates.Citation[[3]], Citation[[4]], Citation[[5]] High plasma levels of catabolic hormones in ARF, e.g., glucagon and catecholamines and the end products of protein catabolism as urea, creatinine, guanidine, indole, sulfate, and cyanate, play significant role in development of toxicity in various organs.Citation[[6]], Citation[[7]] The existence of hormone misbalance, lipid peroxidation, and increased protease activity leads to hypercatabolic state and increases muscle proteolysis. The liver takes up free amino acids arising from skeletal muscle protein breakdown where they are being metabolized through gluconeogenesis and ureagenesis.Citation[[8]], Citation[[9]] Liver is the main organ for detoxification of ammonia, which is the terminal product of protein and amino acids catabolism.
Bioflavonoids are natural substances found in tea, wine, vegetables, fruit, coffee, and cocoa. Epidemiological studies suggest their beneficial effects both on health and in prevention of diseases such as coronary heart disease, osteoporosis, atherosclerosis, cancer, and viral infection.Citation[[10]]
The aim of this study is the investigation of possible beneficial effect of quercetin on arginase activity and uremia level in glycerol-induced kidney failure.
Methods
Male Sprague Dawley rats weighing 250–300 g were used in this experiment. The animals were divided into four groups of six animals each:
Control group, treated with saline, 0.9% sodium chloride solution sc.
For the purpose of ARF induction the rats were treated by 50% glycerol solution in a dose of 8.0 mL/kg im (Gly-ARF).
Quercetin treated rats (Q). Quercetin was given to one group of animals in a dose of 20 mg/kg sc.
Quercetin-Glycerol-ARF (Q-Gly-ARF). In this group rats were pretreated by quercetin in a dose of 20 mg/kg 2 h before glycerol. The rats were on standard laboratory feed and water ad libitum.
The animals were killed in Ketalar anesthesia by decapitation after 48 h. Urea and creatinine levels were measured by standard biochemical analyses (SYNCHRON, CX3, Beckman) in heparinized blood plasma obtained from aorta abdominalis. Livers were quickly removed, washed in cold isotonic NaCl and homogenized.
The arginase activity was measured in liver homogenates according to the method of Porembska and KedraCitation[[11]] on the basis of formed ornithine. The enzyme activity was expressed as microM of ornithine on mg of proteins. Tissue proteins were determined by the method of Lowry et al.Citation[[12]]
The statistical significance of differences was evaluated by Student's t test. The level of significance was defined as p<0.05. Results are expressed as means ± SD.
Results
Results of this study show that glycerol induces acute renal failure in the rats. Blood levels of urea and creatinine were significantly increased in blood plasma of treated rats compared to control group, p<0.001 ().
Figure 1. Comparison of urea plasma levels between experimental groups of animals (series 1 are means ± series; 2 are SD). 1, Control group of animals; 2, Glycerol-induced acute renal failure (Gly-ARF), p<0.001 compared to control group; 3, Group of animals treated with quercetin; 4, Group of Gly-ARF animals pretreated with quercetin (Q-Gly-ARF), p < 0.01 compared to Gly-ARF group.
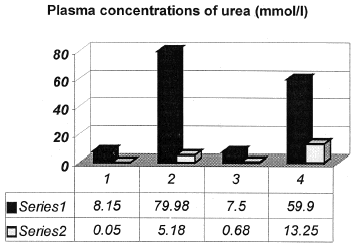
Pretreatment with quercetin decreases blood levels of urea and creatinine compared to G-ARF group, p<0.01 ().
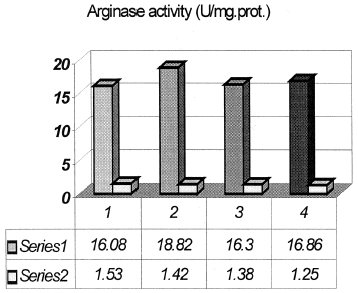
In the same time liver arginase activity was elevated in Gly-ARF, p<0.01. Pretreatment with quercetin leads to decrease of enzyme activity, p<0.05, compared to Gly-ARF group ().
Figure 2. Plasma levels of creatinine (series 1 are means ± series 2 are SD). 1, Control group of animals; 2, Glycerol-induced acute renal failure (Gly-ARF), p < 0.001 compared to control group; 3, Group of animals treated by quercetin; 4, Group of Gly-ARF animals pretreated with quercetin (Q-Gly-ARF), p < 0.01 compared to Gly-ARF group.
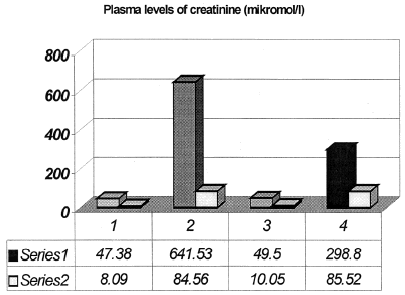
Figure 3. Liver arginase activity (series 1 are means ± series 2 are SD). 1, Control group of animals; 2, Glycerol-induced acute renal failure (Gly-ARF), p < 0.01 compared to control group; 3, Group of animals treated by quercetin; 4, Group of Gly-ARF animals pretreated with quercetin (Q-Gly-ARF), p < 0.05 compared to Gly-ARF group.
Discussion
Flavonoids are phenol components present in various food products. They have beneficial effects both on health and various human diseases. Quercetin and other flavonoids have been shown to have many clinical functions including: anti-inflammatory, vasodilatatory, antiallergenic, hypocholesterolemic, platelet and mast cell stabilization, antihypertensive, antiarrhythmic, and hepatotropic effects.Citation[[13]] In addition to specific effects, bioflavonoids are antioxidants.Citation[[14]]
The potential mechanisms of glycerol induced ARF in the setting of rhabdomyolysis may contribute to renal vasoconstriction, filtration failure, activated endotoxin cytokine cascade, hemeproteins scavenge NO, and increase kidney lipid peroxidation rate.Citation[[15]], Citation[[16]], Citation[[17]] So, we have postulated that flavonoid, quercetin, may be beneficial in experimental model of glycerol-induced ARF.
Results obtained in this study indicate that quercetin, by its influence on arginase activity and plasma levels of urea and creatinine may be beneficial in acute renal failure. The explanation for this effect may be in connection with its ability to act as antioxidant, vasorelaxing and anticatabolic.
Acute renal failure is a serious stress syndrome followed by increased tissue injury as a result of free radicals mechanisms, increased proteolytic activity and tissue hypoxia. Effects of quercetin on arginase activity and uremia may be a key mechanism for its beneficial effect. The depressed consumption of arginine in urea synthesis may provide additional substrate for protein synthesis or/and NO synthesis. The increase of renal regeneration and the decrease of vascular resistance may be the mechanism for restoring of renal function.
Iron-induced oxidative stress has generally accepted mediator of tissue damage. Hem pigment costs have been noted in myoglobinuric ARF. Within proximal tubular cells, the porphyrin ring is catabolized releasing its iron, which is then transferred to ferritin. It greatly facilitates free radical production and may itself become a free radical.Citation[[18]] The quercetin acts as a potent inhibitor of lipid peroxidation in ARF. The most probable mechanisms of lipid peroxidation inhibition by quercetin are: halation of iron, scavenging of free radicals with the formation of less effective radicals, or the increase of GSH content in the liver.Citation[[14]], Citation[[19]], Citation[[20]]
The obtained results show that liver arginase activity is elevated in glycerol-induced acute renal failure. Considering that the level of hepatic urea synthesis is dependent of the availability of substrates, increased arginase activity occurs as a consequence of accelerated protein breakdown.Citation[[21]], Citation[[22]] Interleukin I or some polypeptides in the azotemic plasma promote proteolysis by increasing the production of prostaglandin E.Citation[[23]]
Except for dietary and hormonal regulation, some other factors may be involved in regulation of arginase activity like growing factors and cytokines. The increased expression of cytokines, TNF-a and IL-1 in experimental and human kidney disease is associated with acute tissue damage.Citation[[24]] Beneficial effect of quercetin in G-ARF can be interpreted as a result of its effect on the release of hormones and on the increase of the amino acids free pool for anabolic processes.Citation[[25]], Citation[[26]]
l-Arginine is the precursor not only for urea synthesis but also for synthesis of proteins and other important compounds such as creatinine, nitric oxide, agmatine, polyamines, proline and glutamic acid. Arginine is the common substrate for arginase and nitric oxide synthetase (NOS). In glycerol-induced acute renal failure decreased glomerular nitric oxide production plays an important role in the decrease of renal function.Citation[[27]] Activated NOS pathway and resulting vasodilatation may be one of the mechanisms of beneficial effect of quercetin. NO controls normal renal hemodynamics, the glomerular microcirculation, and renal salt excretion.Citation[[28]] So, the clinical application of quercetin in acute renal failure requires further investigation.
References
- Abuelo J.G. Renal failure caused by chemicals, foods, plants, animal venoms, and misuse of drugs. Arch. Intern. Med. 1990; 150: 505–510
- Schwarz A. Analgesic-associated nephropathy. Klin Wochenschr. 1987; 65: 1–16
- Graetz G. Glucagon stimulation of citrulline formation in isolated hepatic mitochondria. Arch. Biochem. Biophys. 1977; 178: 19–25
- Husson A., Vaillant R. Effects of glucocorticoids and glucagon on argininosuccinate synthetase, argininosuccinase and arginase in fetal rat liver. Endocrinology 1982; 110: 227–232
- Saheki T., Katsunuma T., Sase M. Regulation of urea synthesis in rat liver. J. Biochem. 1977; 82(2)551–558
- Giordano G., de Santo N.G., Senatore R. Effects of catabolic stress in acute and chronic renal failure. Am. J. Clin. Nutr. 1978; 312: 1561–1578
- Wills M. Uremic toxins, and their effect on intermediary metabolism. Clin. Chem. 1986; 3: 5–13
- Clowes G.H.A., George B.C., Jr., Ville C.A., Jr., Saravis C.A. Muscle proteolysis induced by circulating peptide in patients with sepsis or trauma. N. Eng. J. Med. 1983; 308: 545–552
- Horl W.H., Stepinski J., Heidland A. Carbohydrate metabolism and uremia-mechanisms for glycogenolysis and gluconeogenesis. Clin. Wochenschr. 1980; 58: 1051–1064
- Mukhtar H., Ahmad N. Tea polyfenols: prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000; 71: 1698S–1702S
- Porembska Z., Kedra M. Early diagnosis of myocardial infarction by arginase activity determination. Clin. Chim. Acta. 1975; 60: 355–361
- Lowry H.O., Rosenbrough J.N., Far J.A., Randall J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951; 193: 265–275
- Formica V.J., Regelson W. Review of the biology of quercetin and related bioflavonoids. Fd. Chem. Toxic. 1995; 33: 1061–1080
- Robak J., Gryglewski J.R. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988; 37: 837–841
- Zager R.A. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996; 49: 314–326
- Yamada T. Studies on the mechanisms of renal damages induced by nephrotoxic compounds. Nippon. Hoigaku. Zasshi. 1995; 49(6)447–457
- Heyman S.N., Rosen S., Fucho S., Epstein F.H., Brezis M. Myoglobinuric acute renal failure in the rat. A role for medullary hypoperfusion, hypoxia and tubular obstruction. J. Am. Soc. Nephrol. 1996; 7: 1066–1074
- Zager R.A. Iron, heme oxygenase and glutathione. Effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995; 48: 1624–1634
- Cvetkovic T., Vlahovic P., Savic V., Pavlovic D., Kocic G., Djordjevic V.B. Quercetin protects liver from oxidative stress induced by glycerol. J. Hepatology (abstracts) 2000; 32(2)214
- Abull-Ezz R.S., Walker D.P., Shah V.S. Role of glutation in an animal model of myoglobinuric acute renal failure. Proc. Natl. Acad. Sci. USA 1991; 88: 9833–9837
- Horl W.H., Stepinski J., Ganters C., Horl M., Heidland A. Evidence for the participation of proteases on protein catabolism during hypercatabolic renal failure. Klin. Wochenchr. 1981; 59: 751–759
- Wanner C., Schollmeyer F., Horl W.H. Urinary proteinase activity in patients with multiple traumatic injuries, sepsis or acute renal failure. J. Lab. Clin. Med. 1986; 108: 224–229
- Baracos V., Rodemann H.P., Dinarello C.A., Goldberg A.L. Simulations of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). N. Eng. J. Med. 1983; 308: 553–558
- Ketteler M., Border W.A., Noble N.A. Cytokines and arginine in renal injury and repair. Am. J. Physiol. 1994; 267: F197–F207
- Hif C.S., Howell S.L. Effects of flavonoids on insulin secretion and 45Ca++ binding in rat islets of Langerhans. J. Endocrinol. 1985; 107: 1–8
- Kawaida K., Matsumoto K., Shimazu H., Nakamura T. Hepatocyte growth factor prevents acute renal failure of accelerates renal regeneration in mice. Proc. Natl. Acad. Sci. USA 1994; 91(10)4357–4361
- Valdivielso J.M., Lopez-Novoa J.M., Eleno N., Perez Barriocanal F. Role of glomerular nitric oxide in glycerol-induced acute renal failure. Can. J. Physiol. Pharmacol. 2000; 78: 476–482
- Kone B.C., Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am. J. Physiol. 1997; 272: F561–F578
