Abstract
Background. The critically ill patients may require mechanical ventilation, cardiac mechanical support, and other types of critical support. Extracorporeal membrane oxygenation (ECMO) is a supportive therapy, which provides good cardiopulmonary and end-organ support. Continuous renal replacement therapies (CRRT) exhibit important advantages in terms of clinical tolerance and blood purification. This investigation aims to evaluate the acute renal failure in cardiac patients under ECMO, and assess the effect of combining these two technologies, ECMO and CRRT. Methods. Between 12 1998 and 06 2001, 10 adult cardiac patients were treated on ECMO. Five of them were treated with both ECMO and CRRT. The clinical outcomes were retrospectively analyzed. Results. Of the 10 patients studied, five were men and five were women. The mean age of survivors and non-survivors was 37.00 ± 14.54 years and 46.17 ± 7.41 years, respectively. The overall mortality rate was 60%. Survivors did not differ significantly from non-survivors in age or gender. The APACHE II scores on the first day of ECMO support between survival and non-survival were 19.00 ± 9.38 and 24.67 ± 3.50 (P value = 0.392) (Table ), which demonstrates no significant differences too. The cause of death in most patients was related to organ system failure during the 24 h immediately before ECMO started. Five patients with acute renal failure treated by CRRT were eventually died. The median and mean survival in this group on CRRT was 40.50 ± 18.07 h and 92.60 ± 60.50 h. Conclusion. We conclude that mortality rate for acute renal failure in cardiac patients under ECMO continues to be high. Our data suggest that acute renal failure is generally a part of multiorgan failure. This unique form of acute renal failure, causes generalized edema and fluid overload despite still low serum creatinine and azotemia, and deteriorates rapidly to death. From this study shows, advanced cardiac failure may need more aggressive and early initiation of ECMO support before acute renal failure develops. Acute renal failure in advanced heart failure under ECMO support means a grave sign, need aggressive heart transplantation therapy as soon as possible. Combination of CRRT and ECMO might serve an alternative therapy bridging the temporary replacement treatment and heart transplantation in advanced cardiac patients.
Introduction
The critically ill patients may require mechanical ventilation, cardiac mechanical support, and other types of critical assistance. Extracorporeal circulation with a mechanical pump oxygenator offers temporary life support during cardiac operations. As early 1937, John Gibbon proposed the concept of cardiopulmonary bypass to treat severe pulmonary embolism.Citation[[1]], Citation[[2]] Modifications to the techniques and devices allow prolonged extracorporeal circulation in the intensive care unit (ICU), commonly referred to as extracorporeal membrane oxygenation (ECMO) or extracorporeal life support (ECLS).Citation[[3]] It is a supportive therapy used to provide effective cardiopulmonary and end-organ support.
Mild renal function impairment and antidiuresis are common during ECMO support.Citation[[3]] In addition, excessive bleeding frequently occurs when the patients is placed on ECMO, is often life-threatening, and requires the avoidance of anticoagulants with the rapid and massive administration of blood products.Citation[[4]] Although control of coagulation has improved, significant bleeding remains a problem. At this time, no researchers have developed innovative strategies to reduce these problems.Citation[[3]], Citation[[5]] Most of the acute renal failure develops in the first 24 h after ECMO support and some cases are oligoric to anuric type. The acute renal failure is a manifestation of multiple organ system failure due to acute decompensated heart failure, sepsis, aggravated by hemolysis, and activation of complement system during ECMO support.Citation[[3]], Citation[[6]], Citation[[7]] Most of them develop acute pulmonary edema soon after ECMO support due to massive blood transfusion, advanced heart failure, and retrograde non-pulsatile flow during ECMO support increased left ventricular afterload. Most of the other studies agree that acute renal failure, which develops during ECMO support, is associated with poor outcome.Citation[[3]], Citation[[5]], Citation[[7]], Citation[[8]] These may due to accumulation of extravascular water, leading to interstitial overload, impairs oxygen transport through tissues, increased extravascular lung water volume with impairments of O2 transport. Increase of water is itself associated with subsequent organ dysfunction, especially in heart, lungs, and brain.Citation[[6]]
Continuous renal replacement therapies (CRRT) exhibit important advantages in terms of clinical tolerance and blood purification. Continuous renal replacement therapies can proceed simultaneously without anticoagulation and remove plasma water to enable safer transfusion of blood products.Citation[[4]] This study aims to evaluate acute renal failure in cardiac patients under ECMO support, and assess the effect of combining these two technologies, ECMO and CRRT.
Patients And Methods
Patient Information and Management
Between December 1998 and June 2001, 10 adult cardiac patients were treated on ECMO. Five of them developed acute renal failure and were treated with both ECMO and CRRT.
Inotropics were used during the support period to maintain contractility and reduce interventricular blood stasis. An intraaortic balloon pump (IABP) was inserted in most patients to reduce afterload, improve coronary perfusion, and to add a pulsatile component to the circulation. After initiating ECMO, the extent of mechanical ventilation is reduced. Systemic anticoagulation was maintained with continuously infused heparin, to maintain the whole blood activated clotting time (ACT) of 160–180 s. Bleeding was managed by decreasing heparin to achieve an ACT of 140–160 s, and infusing blood products as needed including fresh frozen plasma and cryoprecipitate. Packed red blood cells and platelet were transfused to maintain a hemoglobin concentration of approximately 100 g/L and platelet concentration of approximately 80,000–100,000/µL.
Venoarterial access was established by direct cut down. Drainage typically was accomplished from the right or left femoral vein, whereas arterial access usually was gained through the right or left femoral arteries (). Extracorporeal blood flow (which determines the amount of artificially supplied oxygen) is initially set to 4–4.5 L/min (maximal support with 60 mL/kg). After oxygenation and CO2 removal the blood went back to the body. Ventricular function was monitored at the bedside by echocardiography to allow progressive assessment of myocardial recovery, to exclude intracardiac clot and provide useful information regarding myocardial contractility and ventricular filling during the weaning process. As ventricular recovery progresses, BioPump flow was gradually reduced. Patients were weaned off ECMO when extracorporeal blood flow attained ≤1.0 L/min.
Figure 1. Combination of continuous renal replacement therapies (CRRT) and extracorporeal membrane oxygenation (ECMO) circuit. Drainage is accomplished from the femoral vein, whereas arterial access is gained through the femoral arteries. Continuous renal replacement therapy is incorporated in the ECMO circuit. Arterial drainage of CRRT is coming out before BioPump, passes through the CRRT dialyzer, and goes back to ECMO circuit post-BioPump and before oxygenator. Abbreviation: A = arterial; V = venous.
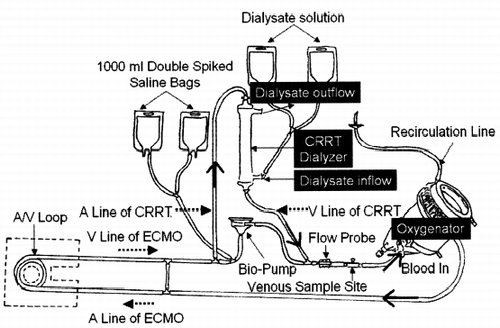
Diuretics were administered if needed to generate adequate urine output and remove excess fluid to maintain the patient at dry weight (preillness weight). If negative fluid balance could not be achieved with diuretics, or acute renal failure developed, continuous renal replacement therapy was performed with a device incorporated in the ECMO circuit. Arterial drainage of CRRT was coming out before BioPump, passed through the CRRT dialyzer, and went back to ECMO circuit post-BioPump and before oxygenator (). Continuous renal replacement therapy was employed to regulate intravascular volume and overall fluid balance, enable the rapid administration of blood products without inducing volume overload, correct azotemia, acid–base, and electrolyte imbalance. The clinical outcomes were retrospectively evaluated.
Definitions
Acute renal failure was defined as sudden sustained decline in glomerular filtration rate, often associated with azotemia, serum creatinine level up to 2.0 mg/dL, oligoria (daily urine amount <500 mL/day), after correcting prerenal cause, mechanical obstruction, gastrointestinal bleeding, and drugs effect.Citation[[7]] The APACHE II raw scores were calculated as described elsewhere.Citation[[9]] summarizes criteria for failure of cardiovascular, pulmonary, neurological, hematological, hepatic, and gastrointestinal systems, which were described previously. Coma due to drug overdose and diabetes mellitus must be excluded for neurological failure. The number of organ system failure included acute renal failure itself.
Statistical Analysis
Continuous variables are summarized by means, standard deviations, and comparisons, evaluated by the Wilcoxon Rank Sum test for unequal variance. The Fisher's Exact test was used to compare categorical variables. p values less than 0.05 were considered statistically significant. All data were entered into a database and analyzed using the Statistical Package for the Social Sciences (SPSS, Version 10.0, Chicago, IL) for Windows.
Results
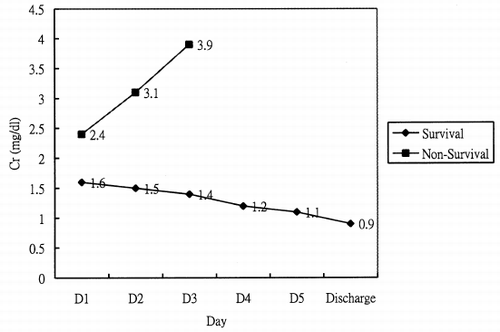
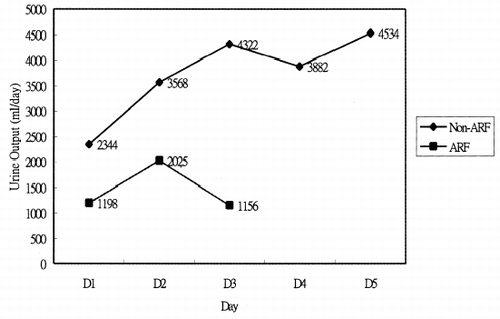
Of the 10 patients with advanced heart failure studied, five were men and five were women. The causes of heart failure were myocarditis, post-cardiotomy cardiogenic shock, and primary graft failure post heart transplantation (). The mean age of survivors and non-survivors was 37.00 ± 14.54 years and 46.17 ± 7.41 years (). The overall mortality rate was 60%. Survivors and non-survivors did not differ significantly in age or gender (). The APACHE II scores on the first day of ECMO support between survival and non-survival were 19.00 ± 9.38 and 24.67 ± 3.50 (p = 0.392) (), which demonstrates no significant differences too. The cause of death in the majority of patients was related to organ system failure during the 24 h immediately preceding the initiation of ECMO. Five patients with acute renal failure treated by CRRT were all died. Acute renal failure mainly developed in the first or second day, related to underlying advanced heart failure. and display the mean creatinine level and daily urine amount between acute renal failure and non-acute renal failure. The mean creatinine level among non-acute renal failure patients is slightly exceeded the baseline at the start of ECMO (1.6 ± 0.34 mg/dL), but the level did not deteriorate progressively (), and returned to baseline after weaning ECMO support. The urine amount is maintained and no acute pulmonary edema developed during the course of treatment and ECMO support (). In the acute renal failure group, the serum creatinine level began to increase in the first 24 h after ECMO support, with mean creatinine level elevated to 2.4 ± 0.67 mg/dL (). Some are non-oligoric type acute renal failure, but some are oligoric to anuric type acute renal failure. Most of them develop acute pulmonary edema and needed continuous renal replacement therapy (). The median and mean survival in this group on continuous renal replacement therapy was 40.50 ± 18.07 h and 92.60 ± 60.50 h.
Table 1. Causes of advanced heart failure
Table 2. Differences between survivor and non-survivor in advanced heart failure patients on ECMO
Table 3. Criteria for organ system failure
Discussion
Acute decompensated heart failure patients are often heterogenous on admission. These acute symptoms are caused by acute or chronic overloading of the heart due to arterial hypertension, coronary heart disease, and/or a reduction in ventricular mass after myocardial infarction.Citation[[10]] The timely recognition and treatment of cardiogenic shock are crucial in reducing the incidence of death.Citation[[10]], Citation[[11]] Patients with cardiogenic shock should be treated aggressively with a combination of pharmacologic agents and, when needed, mechanical support devices to stabilize them as early as possible. Mechanical cardiac support is an important component of a multidisciplinary acute decompensated heart failure program. Different circulatory support devices are frequently required if intraaortic balloon counterpulsation does not provide sufficient support or resolve acute heart failure states, such as extracorporeal membrane oxygenation (ECMO).Citation[[5]], Citation[[10]] In 1997 Bartlett published the results of ECMO procedures reported to the Extracorporeal Life Support Organization (ELSO) registry.Citation[[12]] In the subgroup who underwent ECMO for cardiac failure, the survival rate was 41%. In this small group of patients, ECMO may provide sufficient hemodynamic support to allow recovery from reversible myocardial injury.Citation[[4]]
To our knowledge, no investigation has focus on the renal function with ECMO support. Our investigation indicates that the creatinine concentration in non-acute renal failure patients rises to 1.6 ± 0.34 mg/dL, and returns to baseline after ECMO support is removed (). This mild elevation of creatinine concentration may due to hemolysis, non-pulsatile retrograde renal perfusion, and activation of complement system during ECMO support.Citation[[3]], Citation[[6]], Citation[[7]] The kidney is very sensitive to these effects and cause mild to moderate antidiuresis, but diuretics easily overcome this. No acute pulmonary edema developed during ECMO support in non-acute renal failure patients, even with massive blood product transfusion.
In the acute renal failure group, the serum creatinine level rises in the first 24 h after ECMO support, with mean creatinine level is up to 2.4 ± 0.67 mg/dL (). Some are oligoric to anuric type acute renal failure. The acute renal failure is a manifestation of multiple organ system failure, relating to underlying advanced decompensated heart failure and sepsis, aggravated by hemolysis and activation of complement system during ECMO support.Citation[[3]], Citation[[6]], Citation[[7]] Most of them develop acute pulmonary edema due to massive blood transfusion, advanced heart failure, and retrograde non-pulsatile flow during ECMO support, which increased left ventricular afterload. Five patients with acute renal failure in this study treated by CRRT were eventually died. Most of the other studies agree that acute renal failure develop during ECMO is associated with poor outcome.Citation[[3]], Citation[[5]], Citation[[7]], Citation[[8]] This outcome may due to accumulate of extravascular water, lead to interstitial overload, impairs oxygen transport through tissues, increased extravascular lung water volume with impairments of O2 transport. Increased water is itself associated with subsequent organ dysfunction, particularly in heart, lungs, and brain.Citation[[6]]
Diuretics are administered if needed to sustain adequate urine output and remove excess fluid to maintain the patient at dry weight (pre-illness weight). Like acute renal failure in burn patients, massive blood transfusion, and aggressive fluid resuscitation cause generalized edema and fluid overload despite still low serum creatinine and azotemia.Citation[[13]] This unique form of acute renal failure complicates early diagnosis in advanced decompensated heart failure with ECMO support. Moreover, in our study, patients' condition deteriorated rapidly to death when acute renal failure developed. Continuous renal replacement therapies was beneficial in regulating of intravascular volume and overall fluid balance and to enabling rapid administration of blood and blood products without the inducting volume overload, and correcting azotemia, acid–base, and electrolyte imbalance.Citation[[6]], Citation[[14]] Continuous renal replacement therapies (CRRT) exhibit important advantages in clinical tolerance and blood purification. Continuous renal replacement therapies can proceed simultaneously without anticoagulation and remove plasma water to enable the safer transfusion of blood products.Citation[[4]], Citation[[6]], Citation[[14]] Continuous high volume venovenous hemofiltration, is not only beneficial for sepsis or septic shock with acute renal failure, but also in states of acute heart failure.Citation[[15]], Citation[[16]]
Of the 10 patients studied, five were men and five were women. The mean age of survivors and non-survivors was 37.00 ± 14.54 years and 46.17 ± 7.41 years (p = 0.394). The overall mortality rate was 60%, which resembles other studies.Citation[[4]], Citation[[5]], Citation[[12]] Survivors and non-survivors did not differ significantly in age or gender (). In addition, the APACHE II scores on the first day of ECMO support between survival and non-survival were 19.00 ± 9.38 and 24.67 ± 3.50 (P = 0.392) (), which demonstrates no significant differences. This is different for other diseases associated with acute renal failure, which the APACHE II Scores correlate well with the clinical outcomes.Citation[[8]], Citation[[17]], Citation[[18]] The prognosis of critically ill patients with ARF requiring dialysis is poor, with mortality ranging from 67.3 to 81% in many studies.Citation[[17]], Citation[[18]] Our study produced a 50% incidence of acute renal failure, but the mortality was high; five patients with acute renal failure treated by CRRT were eventually died. The median and mean survival in this group on CRRT was 40.50 ± 18.07 h and 92.60 ± 60.50 h. The cause of death in the majority of patients was related to number of organ system failure during the 24 h immediately before the start of ECMO (2.75 + 0.50 vs. 4.33 ± 0.52, p = 0.007) (). This study demonstrates that the prognosis of advanced heart failure patients with ECMO is closely related to the number of organ failure. Mortality rate increases in line with the number of failed organs on the first day of ECMO. This finding resembles other studies.Citation[[18]], Citation[[19]], Citation[[20]]
We conclude that the mortality rate for acute renal failure in cardiac patients under ECMO continues to be high. Our findings indicate that acute renal failure is usually a part of multiorgan failure complex. The etiology of acute renal failure is related to underlying advanced decompensated heart failure and sepsis, aggravated by hemolysis and activation of complement system during ECMO support.Citation[[3]], Citation[[6]], Citation[[7]] Most of them develop acute pulmonary edema due to massive blood transfusion, advanced heart failure, and retrograde non-pulsatile flow during ECMO support, which increased, left ventricular afterload. This unique form of acute renal failure, causes generalized edema and fluid overload despite still low serum creatinine and azotemia, and deteriorates rapidly to death. Five patients with acute renal failure in this study treated by CRRT were eventually died. From this study shows, advanced cardiac failure may need more aggressive and early initiation of ECMO support before acute renal failure develop. Acute renal failure in advanced heart failure under ECMO support means a grave sign, need aggressive heart transplantation therapy as soon as possible. Combination of CRRT and ECMO might serve an alternative therapy bridging the temporary replacement treatment and heart transplantation in advanced cardiac patients.
References
- Gibbon J.H. Artificial maintenance of life during experimental occlusion of the pulmonary artery. Arch. Surg. 1937; 34: 1105–1131
- Gibbon J.H. The maintenance of life during experimental occlusion of the pulmonary artery followed by survival. Surg. Gynecol. Obstert. 1939; 69: 602–614
- Kolla S., Awad S.S., Rich R.B. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann. Surg. 1997; 226: 544–566
- Smith C., Bellomo R., Raman J.S. An extracorporeal membrane oxygenation-based approach to cardiogenic shock in an older population. Ann. Thorac. Surg. 2001; 71: 1421–1427
- Megovern G.J., Jr., Simpson K.A. Extracorporeal membrane oxygenation for adult cardiac support: the allegheny experience. Ann. Thorac. Surg. 1999; 68: 655–661
- Journois D. Hemofiltration during cardiopulmonary bypass. Kidney Int. Suppl. 1998; 66: S174–S177
- Mols G., Loop T., Geiger K., Farthmann E., Benzing A. Extracorporeal membrane oxygenation: a ten-year Experience. Am. J. Surg. 2000; 180: 144–154
- Smedira N.G., Blackstone E.H. Postcardiotomy mechanical support: risk factors and Outcomes. Ann. Thorac. Surg. 2001; 71: S60–S66
- Knaus W.A., Draper E.P, Wagner D.P. APACHE II: a severity of disease classification system. Crit. Care Med. 1985; 13: 818–824
- Koerner M.M., Loebe M., Lisman K.A. New strategies for the management of acute decompensated heart failure. Curr. Opin. Cardiol. 2001; 16: 164–173
- Rihner M., Smalling R. Cardiogenic shock. Curr. Treat. Opt. Cardio. Med. 2000; 2: 55–64
- Bartlett R.H. Extracorporeal life support registry report 1995. ASAIO J. 1997; 43: 104–107
- Yap H.J., Chen Y.C., Chung S.S., Fang J.T., Huang C.C. Acute renal failure in burned patients. Acta Nephrologica 2000; 14: 17–23
- Fang J.T., Chen Y.C., Tien Y.C., Huang C.C. Continuous venovenous hemodialysis in multiple organ system failure patients with renal failure. Chang Gung Med. J. 1998; 21: 146–151
- Coraim F.J., Coraim H.P., Ebermann R. Acute respiratory failure after cardiac surgery: clinical experience with the application of continuous arteriovenous hemofiltration. Crit. Care Med. 1986; 14: 714–718
- Honore P.M., Jamez W., Wauthier M. Prospective evaluation of short term, high volume isovolumic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit. Care Med. 2000; 28: 3581–3587
- Chen Y.C., Hsu H.H., Kao K.C., Fang J.T., Huang C.C. Outcomes and APACHE II predictions for critically ill patients with acute renal failure requiring dialysis. Ren. Fail. 2001; 23: 61–70
- Chen Y.C., Chen C.Y., Tien Y.C., Fang J.T., Huang C.C. Organ system failures prediction model in intensive care patients with acute renal failure treated with dialysis. Ren. Fail. 2001; 23: 207–215
- Emory R.W., Joyce L.D., Prieto M., Johnson K., Goldenberg I.F., Pritzker M.R. Experience with the symbion total artificial heart as a bridge to transplantation. Ann. Thorac. Surg. 1992; 53: 282–288
- Adamson R.M., Dembitsky W.P., Reichman R.T., Moreno-Cabral R.J., Darly P.O. Mechanical support: assist or nemesis?. J. Thorac. & Cardiovasc. Surg. 1989; 98: 915–920