Abstract
Background/Aims. The concomitant presence of hyperhomocysteinemia and oxidative stress may represent a determinant factor for the occurrence of vascular alterations and cardiac diseases, the main cause of death among dialysis patients. The aim was to analyze the occurrence of hyperhomocysteinemia and oxidative stress and their possible relationship in dialysis patients. Methods. Antioxidant substances, homocysteine, folate, and vitamin B12 were determined in blood from 32 patients on hemodialysis (HD), 21 patients on peritoneal dialysis (PD), and 12 healthy individuals. Results. Different degrees of hyperhomocysteinemia were observed in all HD patients and in 95% of the PD patients (45.30 ± 24.89 µM in HD and 35.50 ± 26.53 µM in PD). Oxidative stress defined as an imbalance between oxidant and antioxidant forces was observed in all dialysis patients, but was more intense in HD individuals. In this group, lipoperoxidation and protein oxidation were associated with lower concentrations of antioxidants such as erythrocyte vitamin E and vitamin C. Conclusions. Hyperhomocysteinemia and oxidative stress occur in both types of dialysis treatment, possibly contributing to the establishment of complications in these patients.
Introduction
Vascular diseases are the major complication in end-stage renal diseases due in part to traditional risk factors such as hypertension and high plasma cholesterol and triglyceride concentrations. The accumulation of homocysteine in plasma of these patients increases this risk.Citation[[1]] The occurrence of hyperhomocysteinemia (fasting values >13.9 µmol/L) was reported in 83–100% of patients with end-stage renal failure, under dialysis treatment.Citation[[2]], Citation[[3]]
Homocysteine is a non-protein forming, sulfur-containing amino acid whose metabolism is situated between two metabolic routes, i.e., remethylation and transsulfuration. During remethylation homocysteine acquires a methyl group from N-5-methyltetrahydrofolate (MTHF) or betaine to form methionine. The reaction with MTHF occurs in all tissues and depends on vitamin B12, while homocysteine formation from betaine occurs mainly in the liver (and kidneys, for humans). During transsulfuration homocysteine is bound to serine to form cystathionine by an irreversible reaction which depends on pyridoxal phosphate (vitamin B6), with cysteine as the final product.Citation[[4]]
In a study of the causes of hyperhomocysteinemia in chronic renal failure, analyzing the kinetics of total homocysteine, it was concluded that chronic renal failure is associated with a significant reduction (70%) in homocysteine clearance, which in turn is related to reduced amino acid uptake and metabolism by the kidneys, and that this reduced clearance probably accounts for hyperhomocysteinemia in these patients.Citation[[5]]
The physiopathology of hyperhomocysteinemia in chronic renal failure may be classified into three main items: reduced renal homocysteine metabolism (and not reduced excretion) as the main cause, reduced capacity of hepatic clearance due to remethylation or transsulfuration, possibly by the uremic route (fewer cofactors and essential substrates or presence of inhibitory enzymes), and deficiency of vitamin B6 and B12 and folic acid which are required for homocysteine metabolism.Citation[[6]] The negative correlation between serum folate and plasma homocysteine observed in patients on hemodialysis may be a consequence of a major defect in the remethylation route of homocysteine, which may be accompanied by an imperfect transsulfuration step, as shown by methionine-loading tests in these patients.Citation[[3]]
Sulfhydryl compounds serve as electron donors in mixed function oxidation systems, being capable of generating hydrogen peroxide. Hydrogen peroxide is capable of producing hydroxyl radicals, an aggressive reactive oxygen species, which are involved in the altered homocysteine metabolism observed in the oxidative processes occurring during atherogenesis, neoplasia, and aging.Citation[[7]]
It is believed that elevated plasma concentrations of homocysteine initiate oxidation reactions, including the oxidative modification of proteins and fatty acid peroxidation. Thiyl radicals, formed from thiol compounds, can react with polyunsaturated fatty acids through hydrogen transfer and addition of double bonds, causing lipoperoxidation in biological systems depending on the lipophilic radical. Oxidative modification of proteins can lead to the loss of catalytic enzyme activity and the formation of carbonyl groups.Citation[[7]], Citation[[8]]
Recent studies have shown significant correlations between hyperhomocysteinemia and lipid peroxidation in humans both in fasting blood and after oral methionine overload, suggesting “oxidative stress” as the plausible mechanism of action of homocysteine in the increased risk of atherosclerosis and other cardiovascular alterations.Citation[[9]], Citation[[10]]
Based on the renewed interest in the prevalence of hyperhomocysteinemia and oxidative stress in dialysis treatment, the aim of the present study was to determine the occurence of high homocysteine concentrations and the establishment of oxidative stress, mainly demonstrated by lipid and protein oxidation, and a possible association between them, in patients on hemodialysis and on continuous ambulatory peritoneal dialysis (CAPD), with both representing risk factors for the occurrence of cardiac diseases and other disorders in this group.
Materials and Methods
A total of 32 patients on hemodialysis treatment (HD) and 21 on ambulatory peritoneal dialysis (PD) participated in the study. Patients were sorted between those with no infectious or acute inflammatory disease. Patients taking current vitamin B supplements alone or in combination with other vitamins were excluded. The control group consisted of 12 apparently healthy individuals.
Before the experiment, the patients were informed about the purpose of the study and the type of sample to be collected. The study was approved by the Research Ethics Committee of the University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo.
Hemodialysis sessions were carried out with a Baxter 1550 apparatus equipped with reusable polysulfone membranes and type F7/F8 capillaries. Blood flow was maintained at a rate of 250–300 mL/min and the dialysate at a flow rate of 350–500 mL/min. The sessions lasted on average 4 h and were repeated three times a week. Patients on PD were submitted to a scheme of four daily bag changes of dialysis solution (2.0-L bags containing 1.5% glucose or 3 bags of 1.5% plus one of 4.25%).
The major cause of renal failure among patients was hypertensive nephrosclerosis (42.8%), followed by chronic glomerulonephritis (7.1%), adult type polycystic kidney (7.1%), and chronic obstructive pyelonephritis, diabetes mellitus, and renal stones (3.6% each). Chronic renal failure of unexplained causes was observed in 28.6% of the patients studied.
Samples from HD patients were collected on the morning of the day of the dialysis session, while collection from PD patients was scheduled for the first morning hours, after dialysis fluid exchange. The majority of individuals in the HD and control groups were males (62.5 and 58.3%), while in the PD group most patients were females (57.2%), with all groups showing a similar mean age.
Analyses
Fasting blood samples were collected by venipuncture and processed within 1 h under refrigeration.
Plasma and erythrocyte lipoperoxidation was indirectly quantified by the determination of malondialdehyde (MDA) in plasma and erythrocytes using a method based on the TBARS (thiobarbituric acid reactive substances) test. The MDA–TBA adduct was separated and quantified by high-performance liquid chromatography with fluorometric detection.Citation[[11]], Citation[[12]], Citation[[13]]
Protein oxidation (carbonyl groups) was determined by a method based on the introduction of carbonyl groups into the amino acid residues of proteins and the reaction of these groups with specific reagents.Citation[[14]], Citation[[15]]
Folate and vitamin B12 were quantified in plasma with automated immunoassay kits using an IMx type analyzer (Abbott Laboratories, USA). Total Homocysteine was detected using an HPLC kit with fluorometric detection (Chromsystems, Germany). Glutathione peroxidase (GPx) was analyzed using a modification of the method of Maral et al.Citation[[16]] The amount of GPx in the sample was estimated by the consumption of NADPH in the reaction and the consequent reduction in absorbance 5 min after the addition of t-butyl-hydroperoxide. Plasma alpha-tocopherol concentration was determined by a method using HPLC with UV detection.Citation[[17]] Ascorbic acid was also quantified by HPLC with UV detection, using an adaptation of a previously described method.Citation[[18]]
Statistical Analysis
Differences between groups were determined using the nonparametric Kruskal-Wallis test for independent samples and the Dunn post-test. Correlations between the different variables were calculated by the nonparametric Spearman coefficient of correlation. Parametric analysis of variance (ANOVA) and Pearson correlation coefficient were used for the analysis of the anthropometric variables. The level of significance was set at p<0.05 for all tests. STATISTICA® and GraphPad InStat® were the statistical program used to perform tests.
Results
No statistically significant differences in mean age or body mass index (BMI) were observed between the HD, PD, and control groups, or between sexes in each group. In all groups, most individuals showed a BMI within the normal range (18.5–25.0 kg/m2). A deficient BMI was only observed for one patient in the HD group and mild obesity ranging from 16.7–17.7% was observed in all groups. Mean age, BMI, and sex distribution between groups are shown in .
Table 1. Mean (±SD) age and BMI, and sex distribution for the groups studied
Mean treatment duration in the PD group was 13.6 months, with majority (52.3%) being under treatment for a period of 7–30 months. In the HD group, 71.0% of patients presented a treatment duration of 1–5 years, which was significantly longer than that observed for the PD group.
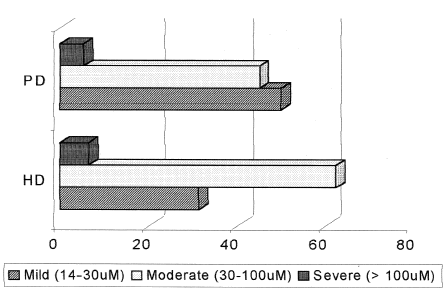
Hyperhomocysteinemia, defined as plasma values above 14.0 µM, was detected in all HD patients and in all but one PD patient, who did not present an elevated amino acid concentration, leading to a 95.2% incidence in the latter group. Folate deficiency (<7.0 nM) was found in 18.7% HD and 19.0% PD patients, and B12 deficiency (<164 pM) was detected in 6.2% in HD and 14.3% in PD patients, with no statistical difference between groups. No statistically significant difference regarding the form of hyperhomocysteinemia was observed between the HD and PD groups. shows the distribution of the different forms of hyperhomocysteinemia among the two patient groups.
No significant differences in mean vitamin B12, plasma vitamin E, or erythrocyte GPx were observed between the HD, PD, and control groups (). HD and PD patients showed similar homocysteine and carbonyl values, which were significantly higher than those observed for the control group, while folate and vitamin C concentrations were lower than those observed for the control group. Mean plasma and erythrocyte MDA levels were similar for the PD and control group and were significantly lower than the values observed for the HD group. Mean erythrocyte vitamin E concentration was significantly lower in the HD group than in the PD group ().
Table 2. Mean (±SD) homocysteine (HCy), folic acid, vitamin B12, oxidative parameters, and antioxidants concentrations of the groups studied
In the HD group, significantly negative correlations were obtained between homocysteine and folate, and between homocysteine and vitamin B12, while a positive correlation was found between homocysteine and vitamin C; no significantly correlations were found between homocysteine and oxidative parameters. The same correlations were observed for the PD group, except for the correlation between homocysteine and folate. Folic acid was negatively correlated with carbonyl groups in PD patients and with plasma MDA in HD patients. These coefficients and other significant correlations between the analyzed parameters are shown in for each group studied.
Discussion
Direct comparison of the basal values obtained for the HD group with those observed for the PD and control groups revealed moderate hyperhomocysteinemia in both dialysis groups, including all patients of the HD group and 20 of the 21 PD patients. The mild and moderate forms were predominant, but plasma values above 100 µM were found in 6.2% of HD and 5.0% of PD patients.
Table 3. Spearman correlation coefficient and significance levels for the correlations obtained for the HD and PD groups
Highly heterogenous results in terms of plasma folate and vitamin B12 concentrations have been obtained in other studies evaluating hemodialysis patients, including folate deficiency in 81% of 18 patients under hemodialysis and vitamin B12 deficiency in 32.8% of hemodialysis patients.Citation[[19]], Citation[[20]] At the other extreme, Tremblay et al.Citation[[21]] did not observe folate deficiency in hemodialysis patients and only 6% were deficient in vitamin B12.
Although several lines of evidence suggest that the kidneys and the efficiency of the metabolic routes play an important role in the control of hyperhomocysteinemia, the type of dialysis may represent an additional factor contributing to this disorder. In a study carried out on 130 patients on hemodialysis and 46 on peritoneal dialysis, hyperhomocysteinemia was more prevalent and more severe in the hemodialysis group, and was negatively associated with folate and vitamin B12 and B6 concentrations. In contrast, the peritoneal dialysis group showed lower homocysteine and higher folate, B12 and B6 concentrations than the hemodialysis group, attributable to reduced losses through the dialysis bag, thus determining the form of hyperhomocysteinemia in these patients.Citation[[22]]
The prevalence of hyperhomocysteinemia was closely similar to that observed in the present study, but the two-dialysis treatments did not differ regarding the form of hyperhomocysteinemia. In addition, no differences in mean vitamin B12 and folate, or in the incidence of vitamin deficiency were observed between groups.
In a study comparing hemodialysis and peritoneal dialysis, hyperhomocysteinemia was observed in 96% of patients on peritoneal dialysis (n = 82) and 97% of patients on hemodialysis (n = 70), with mean homocysteine concentrations being significantly higher in hemodialysis patients. Folic acid and B12 concentrations were normal in most patients and serum folic acid was found to be the determinant factor of the high homocysteine concentration. Folate concentrations within the reference range are insufficient to maintain the homocysteine levels in dialysis patients.Citation[[23]]
The results are similar to those observed in the present study with respect to the incidence of hyperhomocysteinemia during dialysis, but no significant difference in mean values were observed between groups. Homocysteine was negatively correlated with folate and vitamin B12, and was probably influenced by the status of both vitamins.
Taken together, the data show higher lipoperoxidation and protein oxidation in patients submitted to dialysis and a reduction in an important plasma antioxidant, i.e., vitamin C, leading to a picture of chronic “oxidative stress” in these patients. It should be noted that there was a small difference in the time of blood sample collection between the HD and PD groups. Hemo dialysis patients had their samples collected immediately before or during the morning of the hemodialysis session, while blood samples from PD patients were always collected during the morning after the first bag change. Therefore, in contrast to the PD group, the HD group had reached maximum accumulation of uremic substances, completing approximately 48 h without dialysis. Duration of treatments was significantly different between the dialysis groups and can therefore also contribute for different findings between groups.
Several lines of evidence implicate the oxidative process caused by free radicals and the reduction in antioxidant defenses in the complications and comorbidity associated with uremia and hemodialysis. The common route of uremia and hemodialysis is the amplified inflammatory response, in which the initial stimulus triggers the production of cytokines as well as free radicals which, in turn, amplify the reaction, thus forming a vicious cycle.Citation[[24]] The initial stimulus during hemodialysis may be the dialysis procedure itself through the use of less biocompatible membranes, which represents one of the unfavorable factors in hemodialysis treatment compared to peritoneal dialysis.Citation[[25]], Citation[[26]] However, oxidative products are also elevated in uremic patients not receiving dialysis, indicating that oxidative stress can be caused by uremia per seCitation[[27]] The imbalance between oxidant and antioxidant forces, mainly due to vitamin loss during dialysis, may also play an important role.Citation[[28]]
In the present study, the mean plasma carbonyl group concentrations were significantly higher in the HD and PD groups than in the control group, confirming a carboxylic stress in dialysis patients. Factors responsible for the accumulation of carbonyl groups in uremic patients are higher production favored by an oxidative environment and higher retention due to reduced clearance, possibly as a result of impaired urinary excretion and lower detoxification of the compounds due to glutathione homeostasis imbalance.Citation[[27]]
There is strong evidence that oxidative stress is one of the mechanisms involved in the action of hyperhomocysteinemia leading to endothelial damage. Homocysteine is readily oxidized when reaching plasma, mainly as a result of its auto-oxidation into homocysteine, homocysteine disulfide derivatives, and thiolactone–homocysteine. Superoxide radical and hydrogen peroxide are formed during oxidation of the sulfhydryl group, thus initiating lipoperoxidation both on the endothelial surface and in plasma lipoprotein particles.Citation[[29]]
Folic acid itself may have an antioxidant effect counteracting the oxidative damage induced by the accumulation of homocysteine.Citation[[30]] This theory may explain in part the correlations observed between folate and MDA and between folate and carbonyl in HD and PD patients, respectively, but further studies are required to confirm these speculations.
A study using 100 fasting blood samples obtained from healthy men reported one of the first in vivo proofs of the fact that plasma homocysteine concentrations are associated with increased lipoperoxidation, as determined by plasma F2-isoprostane analysis.Citation[[10]] Other authors, using methionine overload in healthy individuals, found positive correlations between homocysteine and carbonyl groups and MDA, and negative correlations between homocysteine and antioxidant vitamins, 8 h after the load, indicating the pro-oxidative effect of high homocysteine concentrations in vivo.Citation[[31]]
Our results demonstrate the simultaneous presence of homocysteine accumulation and lipid and protein oxidation in dialysis patients, especially in hemodialysis; PD patients presented mild hyperhomocyteinemia and slightly eleveted protein oxidation levels. The oxidative stress observed in HD patients may be caused by multiple factors associated with both renal failure and processes triggered by the dialysis procedure and low levels of folate may work in association with these events. The mechanisms involved still need to be investigated, such as the impact of supplementation with folic acid and other vitamins, specially antioxidants, on the reduction of homocysteine concentrations and the possible alteration of the oxidative state, which might lead to a reduction in the morbidity and mortality due to cardiovascular diseases in dialysis patients.
References
- Kunz K., Petitjean P., Lisri M., Chantrel F., Koehl C., Wiesel M.L., Cazenave J.P., Moulin B., Hannedouche T.P. Cardiovascular morbidity and endothelial dysfunction in chronic hemodialysis patients: is homocyst(e)ine the missing link?. Nephrol. Dial. Transplant. 1999; 14(8)1934–1942
- Bostom A.G., Shemim D., Lapane K.L., Hume A.L., Yoburn D., Nadeau M.R., Bendich A., Selhub J., Rosenberg I.H. High dose B-vitamin treatment of hyperhomocysteinemia in dialysis patients. Kidney Int. 1996; 49: 147–152
- Van Guldener C., Janssen M.J.F.M., de Meer K., Donker A.J., Stehouwer C.D. Effect of folic acid and betaine on fasting and postmethionine-loading plasma homocysteine and methionine levels in chronic haemodialysis patients. J. Internal Med. 1999; 245: 175–183
- Bostom A.G., Lathrop L. Hyperhomocysteinemia in end-estage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997; 52: 10–20
- Guttormsen A.B., Ueland P.M., Svarstad E., Refsum H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney Int. 1997; 52: 495–502
- Dennis V.W., Robinson K. Homocysteinemia and vascular disease in end-stage renal disease. Kidney Int. 1996; 50: S11–S17
- Olszewski A.J., McCully K.S. Homocysteine metabolism and the oxidative modification of proteins and lipids. Free Radical Biol. Med. 1993; 14: 683–693
- Schöneich C., Dillinger U., von Bruchhausen F., Asmus K.D. Oxidation of polyunsaturated fatty acids and lipids through thiel and sulfonyl radicals: reaction kinetics, and influence of oxygen and structure of thiel radicals. Arch. Biochem. Biophys. 1992; 292: 456–467
- Domagala T.B., Libura M., Szczeklik A. Hyperhomocysteinemia following oral methionine load is associated with increased lipid peroxidation. Thrombosis Res. 1997; 87: 411–416
- Voutilainen S., Morrow J.D., Roberts L.J., Alfthan G., Alho H., Nyyssonen K., Salonen J.T. Enhanced in vivo lipid peroxidation at elevated plasma total homocysteine levels. Arterioscler. Thromb. Vasc. Biol. 1999; 19: 1263–1266
- Wong S.H., Knight J.A., Hopfer S.M., Zaharia O., Leach C.N., Sunderman F.W. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde–thiobarbituric acid adduct. Clin. Chem. 1987; 33: 214–220
- Fukunaga K., Susuki T., Takama K. Highly sensitive high-performance liquid chromatography for the measurement of malondialdehyde in biological samples. J. Chromatogr. 1993; 621: 77–81
- Fukunaga K., Takama K., Susuki T. High-performance liquid chromatographic determination of plasma malondialdehyde level without a solvent extraction procedure. Anal. Biochem. 1995; 230: 20–23
- Levine R.L., Garland D., Oliver C.N., Amici A., Climent A., Lenz A.G., Ahn B.W., Shalfiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990; 186: 464–478
- Odetti P., Garibaldi S., Gurreri G., Aragno I., Dapino D., Pronzato M.A., Marinari U.M. Protein oxidation in hemodialysis and kidney transplantation. Metabolism 1996; 45: 1319–1322
- Maral J., Puget K., Michelson A.M. Comparative study of superoxide dismutase, catalase, and glutathione peroxidase levels in erythrocytes of different animals. Biochem. Biophys. Res. Commun. 1977; 77: 1525–1535
- Arnaud J., Fortis I., Blachier S., Kia D., Favier A. Simultaneous determination of retinol, α-tocoferol and β-caroteno in serum by isocratic high-performance liquid chromatography. J. Chromatogr. 1991; 572: 103–116
- Rose R.C., Bode A.M. Analysis of water soluble antioxidants by high-pressure liquid chromatography. Biochem. J. 1995; 306: 101–105
- Korzets A., Ori Y., Chagnac A., Weinstein T., Herman M., Zevin D., Malachi T., Gafter U. Erythropoietin, folic acid deficiency and hyperhomocysteinemia: is there a possible relationship in chronically hemodialyzed patients?. Clin. Nephrol. 2000; 53: 48–54
- Chandna S.M., Tattersall J.E., Nevett G., Tew C.J., O'Sullivan J., Greenwood R.N., Farrington K. Low serum vitamin B12 levels in chronic high-flux haemodialysis patients. Nephron 1997; 75: 259–263
- Tremblay R., Bonnardeux A., Geadah D., Busque L., Lebrun M., Ouimet D., Leblanc M. Hyperhomocysteinemia in hemodialysis patients: effects of 12-month supplementation with hydrossoluble vitamins. Kidney Int. 2000; 58: 851–858
- Moustapha A., Gupta A., Robinson K., Arheart K., Jacobsen D.W., Achreiber M.J., Dennis V.W. Prevalence and determinants of hyperhomocysteinemia in hemodialysis and peritoneal dialysis. Kidney Int. 1999; 55: 1470–1475
- De Vecchi A.F., Bamonti-Catena F., Finazzi S., Campolo J., Taioli E., Novembrino C., Colucci P., Accinni R., de Franceschi M., Fasano M.A., Maiolo A.T. Homocysteine, vitamin B12, and serum and erythrocyte folate in peritoneal dialysis and hemodialysis patients. Perit. Dial. Int. 2000; 20: 169–173
- Wratten M.L., Tetta C., Ursini F., Sevanian A. Oxidant stress in hemodialysis: prevention and treatment strategies. Kidney Int. 2000; 58: S126–S132
- Westhuyzen J., Adams C.E., Fleming S.J. Evidence for oxidative stress during in vitro dialysis. Nephron 1995; 70: 49–54
- Mimic-Oka J., Simic T., Djukanovic L., Reljic Z., Davicevic Z. Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin. Nephrol. 1999; 51: 233–241
- Miyata T., Kurokawa K., Strihou C.V.Y. Relevance of oxidative and carbonyl stress to long-term uremic complications. Kidney Int. 2000; 58: S120–S125
- Tetta C., Biasioli S., Schiavon R., Inguaggiato P., David S., Panichi V., Wratten M.L. An overview of haemodialysis and oxidant stress. Blood Purif. 1999; 17: 118–126
- Loscalzo J. The oxidant stress of hyperhomocysteinemia. J. Clin. Invest. 1996; 98: 5–7
- Brattström L., Wilcken D.E.L. Homocysteine and cardiovascular disease: cause or effect. Am. J. Clin. Nutr. 2000; 72: 315–323
- Ventura P., Panini R., Verlato C., Scarpetta G., Salvioli G. Peroxidation indices and total antioxidant capacity in plasma during hyperhomocysteinemia induced by methionine oral loading. Metabolism 2000; 49: 225–228