Abstract
Background. Chronic renal failure patients on hemodialysis are at an increased risk of acquiring hepatitis B infection. Hence vaccination against hepatitis B assumes great importance in these patients. However, the response to hepatitis B vaccination is poor, even when 4 double doses (40 µg) of the vaccine are given. This study was conducted to determine the efficacy of GM-CSF as an adjuvant to hepatitis B vaccine in CRF patients. Methods. CRF patients including both hemodialysis (HD) and non-dialysis (ND) patients were randomized to receive either placebo or a single injection of GM-CSF (in varying doses of 50 µg, 100 µg, 150 µg) a day prior to the 1st dose of recombinant hepatitis B vaccine (40 µg). Three more doses of the vaccine were given at 1, 2, and 6 months. The anti-HBs antibody titres were measured by ELISA at 3 and 7 months. Patients having antibody titres less than 10 IU/L were considered non-responders. The response rate and mean antibody titers were compared between the control (I) and GM-CSF (II) groups. Results. In group I, 31 and 27 patients were available for evaluation at 3 and 7 months respectively. In group II, 33 and 28 patients could be evaluated at the same time points. Within the control group (group I), the response rate in hemodialysis patients (63.6%) was lower as compared to non-dialysis patients (81.2%). The response rate in group II was higher than that in group I at both 3 months as well as 7 months (78.1% vs. 62.3% and 89.3% vs. 74.1%, p = ns). The best response rates in group II were observed when GM-CSF was used in a dose of 150 µg (90.9% at 3 months and 100% at 7 months). The mean antibody titers were also found to be higher in the group II as compared to group I (409.6 vs. 243.9 IU/L, p = 0.01). Conclusion. The results of this randomized, prospective study suggest that: 1. Patients with chronic renal failure should be vaccinated for hepatitis B as chronic renal insufficiency is established. 2. GM-CSF is an effective adjuvant to hepatitis B vaccine in these patients especially when a priming dose of 150 µg is used prior to 1st dose of hepatitis B vaccination.
Introduction
Hepatitis B virus (HBV) along with hepatitis C virus is a formidable cause of chronic liver disease and primary liver cancer worldwide. Hepatitis B infection is a major problem in patients of chronic renal failure (CRF), especially those on hemodialysis (HD), in whom the prevalence of HBV infection has been found to be several fold higher than in the general population. The hepatitis B prevalence in hemodialysis units of India has been reported to range from 7.7–31%.Citation[[1]], Citation[[2]], Citation[[3]] Since the hemodialysis patients acquiring hepatitis B infection are a potential risk to their contacts, prevention of infection through vaccination is of great importance.
Unfortunately, in patients with CRF, hepatitis B (HB) vaccination achieves disappointing seroprotection rates (defined as an anti-HBS titer >10 IU/mL, 1–2 months after the last dose of standard HB vaccination schedule) rates. This poor response to HB vaccine in CRF patients has been attributed to the generalized state of immunodeficiency observed in such patients. In vitro studies have shown a reduction in IL-2 release by peripheral blood mononuclear cells (PBMCs) in CRF.Citation[[4]] Upregulation of IL-2R expression on T-cells and increased serum levels of soluble IL-2R further lead to reduced bioavailability of IL-2 and contribute to the development of immunodeficient state.Citation[[5]] A reduction in the number of TCR/CD3 antigen receptor complex has been reported on CD4 T cells in uremics.Citation[[6]] Both cytokine dependent and cytokine independent T cell functions are defective in renal failure patients. Complement system is also affected by renal failure-plasma levels of Ba, a 33 kD activation product of factor B that suppresses B lymphocyte function in vitro are highly elevated in patients with renal failure.Citation[[7]] CRF patients on hemodialysis have also been shown to have impaired expression of co-stimulatory molecule B7-2 (CD-86) on monocytes,Citation[[8]] thus resulting in inadequate antigen presentation to immune-competent cells. Possibly, this step may be the most significant one in contributing to poor response to vaccination in these patients. To over come this non-responsiveness, 4 doses of twice the standard adult dose i.e., 40 µg of recombinant HB vaccine at 0, 1, 2, and 6 months has been recommended. In spite of this, a substantial proportion of CRF patients do not achieve protective levels of the antibody.Citation[[9]], Citation[[10]], Citation[[11]]
New strategies are being tried to combat this problem of non-responsiveness, including the use of adjuvants like interleukin,Citation[[12]] thymopentin,Citation[[13]] interferon,Citation[[14]] and erythropoietin.Citation[[15]] The most encouraging results seen in many uncontrolled studies have been with the use of granulocyte macrophage-colony-stimulating factor (GM-CSF) as an adjuvant to HB vaccination. The pilot data from our center revealed good efficacy of this agent with seroprotection in 100% of those who received a priming dose prior to the 1st dose of HB vaccination.Citation[[16]]
This prospective, randomized, controlled study was performed to assess the efficacy of GM-CSF as an adjuvant to HB vaccination and to ascertain the optimal dose of the adjuvant.
Patients and Methods
Inclusion Criteria
Patients of CRF with s. creatinine >2.0 mg/dL, creatinine clearance rate (CCR) <50 mL/min either on HD, or conservative management (non-dialysis, ND) were screened to be enrolled into the study. All patients were required to give written informed consent. The institutional and hospital ethical committee approved the study.
Exclusion Criteria
Patients with positive serology for HBsAg, total anti-Hbc, anti HCV, HIV 1 and 2, prior use of any immunomodulatory agents like steroids/vaccine adjuvants in last 6 months, prior HB vaccination or those receiving blood transfusion in previous 6 months were excluded.
The study group finally comprised of 71 patients who were randomized into 2 groups:
Group I (n = 35)—controls, i.e., CRF patients who received only 4 doses of 40 µg recombinant HB vaccination (Enivac-HB, Panacea Biotec) intramuscularly at 0, 1, 2, and 6 months. Of these 35, 17 were on maintenance HD and 18 were ND patients.
Group II (n = 36)—patients, i.e., CRF patients who in addition to the vaccination schedule as above, received a single injection of GM-CSF (Leucomax, Novartis) subcutaneously 1 day prior to the 1st dose of the vaccine (). This group was further randomized into 3 groups of 12 patients each on basis of dose of GM-CSF used:
Each of these 3 groups had 5 HD and 7 ND CRF patients. The individual categories in 2 groups of patients were matched for age, sex and CCR. GM-CSF was administered subcutaneously in the upper arm and patients were kept under observation for 48 h for any adverse events.
All patients were evaluated with hemoglobin, total leucocyte count, serum creatinine, CCR, blood urea at the baseline. The anti-HBs antibody titers were measured in all patients at baseline, at 3 months (i.e., 1 month after 3rd dose of HB vaccine) and at 7 months (i.e., 1 month after completion of vaccination schedule) with commercially available ELISA kits (upper limit of detection of the kit was 1000 IU/L; if titers were >1000 IU/L serial dilution were used). The observer who analyzed the antibody titres was blinded i.e., had no knowledge as to whether the sample under analysis was from a patient who received/did not receive GM-CSF.
The primary end points of the study were:
Efficacy of GM-CSF as an adjuvant to HB vaccination. Patients with anti-HBs titres >10 IU/L were considered responders.
Level of antibody responses to differing doses of GM-CSF.
The secondary end points were:
Comparison of antibody responses in non-dialysis (ND) vs. hemodialysis (HD) group.
Statistical analysis: The response rate to vaccination in different groups was compared using the chi-square or the Fischer's exact test. The difference in the mean antibody responses between 2 groups was calculated using the 2-sample ‘t’ test. A p<0.05 was considered to be statistically significant.
Results
The demographic and biochemical profile is summarized in . The etiology of CRF in majority of the patients, as depicted, was diabetic nephropathy.
Table 1. Demographic and biochemical profile of the study groups.a
Of the 71 patients included in the study, 64 could be evaluated at 3 months. Three patients died (2 in Gp I and 1 in Gp II, all on HD). Three others chose to be referred to centers with transplant facilities and were hence excluded from analysis (2 in Gp I HD and 1 in Gp II HD). One patient in Gp II ND progressed to stage of renal failure requiring hemodialysis, which was instituted at a center equipped with transplant facility. Therefore, of the 64 patients analyzable at 3 months, 31 belonged to Gp I and 33 to Gp II. At the end of the 7 months study period, attrition of 9 more patients occurred. Four died, 2 in each hemodialysis group and 5 ND patients progressed to stage of renal failure requiring hemodialysis, 2 in Gp I and 3 in Gp II, which was instituted at centers enrolling for renal transplantation. Therefore, of the 55 patients analyzable at 7 months, 27 belonged to Gp I and 28 to Gp II. Overall, the response rate to vaccination could thus be analyzed in 64 patients at 3 months and 55 patients at 7 months.
The overall response rate at 3 months and 7 months in the 2 groups of patients is summarized in . The anti-HBs antibody titers seen within patients of group II, receiving 50, 100 or 150 µg GM-CSF is summarized in . GM-CSF use resulted in minor side effects in 9/36 (25%) patients and included fever-4, headache-2, nausea and vomitting-1, arthralgia-1 and erythema at injection site-1. All these side effects were self-limiting.
Table 2. Response rates to hepatitis B vaccination in the two groups at 3 and 7 months
Table 3. Response rates to hepatitis B vaccination in patients receiving 50, 100, or 150 µg GM-CSF
Discussion
The use of various adjuvantsCitation[[12]], Citation[[13]], Citation[[14]], Citation[[15]] and intradermal vaccination.Citation[[17]] has been attempted to get enhanced seroprotection to HB vaccination CRF patients. GM-CSF, possibly the most promising of them all, is a hematopoietic growth factor that induces the proliferation and maturation of precursor cells into granulocyte and macrophage colonies.Citation[[18]] It is a major stimulatory cytokine for the viability, differentiation and function of antigen presenting cells viz., langerhans cells and dendritic cells.Citation[[19]] GM-CSF also increases the expression of MHC class II molecules on the antigen presenting cellsCitation[[20]] and affects secretion of cytokines by the macrophages.Citation[[18]] These immunostimulatory properties of GM-CSF are probably thought to be responsible for the enhanced antibody responses to HB vaccination seen in the pilot studies ().
Table 4. Response to hepatitis B vaccine in hemodialysis patients treated with GM-CSF as an adjuvant
Response Rates to Vaccination with and Without GM-CSF
In the present study, the 2 groups of patients had comparable demographic profile and severity of renal dysfunction. As is evident from , the response rate to HB vaccination was higher in patients receiving GM-CSF compared to controls both at three months (78.1% vs. 61.3%) and at seven months (89.3% vs. 74.1%), though the differences were not statistically significant. Among the group II patients the patients receiving 150 µg GM-CSF had the highest response rate compared to the 50 µg and 100 µg GM-CSF groups respectively at three months (90.9% vs. 63.6, 81.8%) and seven months (100% vs. 77.8, 88.9%). It is significant to note that all patients receiving 150 µg GM-CSF were seroprotected at seven months. The responses in the 50 µg GM-CSF group were comparable to the control group respectively at three months (63.6% and 61.3%) and seven months (77.8% and 74.1%). This suggests that a dose of 50 µg GM-CSF prior to HB vaccination is of little use. Evans et al. similarly demonstrated that 40 and 80 µg GM-CSF given intramuscularly had response rates similar to the placebo group.Citation[[26]]
Kher et al. used 150 µg GM-CSF prior to each dose of HB vaccination found a 92% response after three doses of vaccination.Citation[[27]] This suggests that a single dose of 150 µg GM-CSF may be optimal and that multiple doses may not offer any added advantage.
Response Rates in Non-dialysis and Dialysis Patients
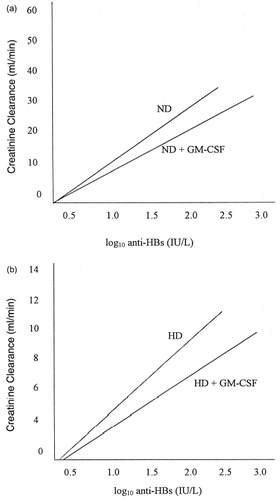
Another significant observation is this study was the marked difference in response rates observed in the ND and HD patients. In the control group, the response rates in the ND and HD population were 66.7% vs. 53.8% and 81.2% vs. 63.6% at three and seven months (p = ns) respectively. In the GM-CSF group the response rates were 84.2% vs. 69.2% and 94.1% vs. 81.8% at three and seven months (p = ns) respectively. Although statistical significance could not be attained (possibly due to small sample size of individual groups), the trends are quite apparent. The correlation between the CCR and anti-HBs titers was linear in both the ND as well as the HD group. The use of GM-CSF shifts the antibody response curve to the right, at any given level of CCR (a and b). Other workers have made similar observations. Bommer et al. reported a response rate of 65% in patients with mild renal insufficiency compared to 54% in HD patients.Citation[[28]] Dukes et al. found 84% response in ND patients compared to 52% in dialysis patients.Citation[[29]] These observations strongly support the practice of vaccination right at the stage when renal impairment is first diagnosed.
Figure 2. Relationship between creatinine clearance and anti-HBs responses in patients treated conservatively (not dialyzed, ND) (2a) or with hemodialysis (HD) (2b). The antibody response curve is shifted to the right with the use of GM-CSF as a vaccine adjuvant. The data represent the antibody titers at 3 months.
Mean Antibody Levels
Not only is the seroprotection rate of patients important, but also of considerable significance is the degree of response (i.e., the actual antibody titer). A higher antibody response has also been suggested to be a determinant of the sustainability of the seroprotection. It is quite clear from our results that patients receiving GM-CSF had higher mean anti-HBs levels at seven months than the patients who did not receive the priming dose of the adjuvant (409.6 vs. 243.7 IU/L, p = 0.014). At three months the mean antibody levels after the use of GM-CSF were higher, but statistical significance could not be achieved (198.9 vs. 127.4 IU/L, p = ns). The use of GM-CSF also resulted in a more rapid seroprotection with 19 of 31 (61%) patients in group I seroprotected at three months vis-à-vis 25 of 32 (78%) in group II. Though the numbers did not reach a statistical significance but a type II error cannot be ruled out. As previously mentioned, the antibody levels were determined by the severity of renal insufficiency and were higher in ND patients than those on HD ().
Table 5. Comparison of mean antibody titers in study groups
Overall response rate was highest in patients aged <40 years (15/16 i.e., 93.8%) compared to patients in 40–60 years age group (24/29 i.e., 82.8%) and least in patients over 60 years (6/10 i.e., 60%) signifying a declining response to vaccination with increasing age. This trend was observed in group I as well as in group II. Group II patients aged less than 40 years showed 100% response (8/8) while the poorest response was seen in group I patients aged more than 60 years (2/5, 40%). The response rates were not observed to be sex dependent or dependent on the etiology of CRF. Another significant observation was that all patients (i.e., 9/9 or 100% with a CCR >35 mL/min achieved seroprotective antibody levels in comparison to a 70% response in patients with CCR <10 mL/min (14/20).
The potential drawback of this study is that it does not elucidate the mechanisms involved in potentiation of the vaccine response by the use of GM-CSF. Also, due to unavoidable reasons, the vaccination efficacy could not be assessed in 16 patients (22%), partly due to non-availability of transplant facilities at our center. A potential limitation of a study enrolling seriously ill patients (as those in this study) is that the patient profile may change with time and the requirement for renal replacement therapy may set in. This situation arose in some of our patients, who chose to be dialyzed at centers equipped with transplant facility and hence could not be included in the final analysis.
To conclude, GM-CSF used as an adjuvant to HB vaccination offers enhanced responsiveness with 100% of patients achieving seroprotective titres at seven months. The use of this agent also results in a more rapid seroprotection, which is of potential benefit in patients on HD. The optimum dose of GM-CSF observed in this study was 150 µg given as a single dose one day prior to vaccination. GM-CSF administration also resulted in significantly higher titres of protective antibodies. At the present time, the cost-effectiveness of this regimen needs to be assessed in the light of the cost, discomfiture and morbidity of repeated doses (and varying routes, e.g., intradermal) of the vaccine which the non-responders to standard schedule have to undergo. This is in addition to the cost of repeated tests for the anti-HBs titers, which are performed to assess seroprotection after each phase of vaccination. In our unpublished experience, the non-responder patients treated with only a greater number of vaccine doses, attain low antibody titers. It is imperative to initiate vaccination as early as possible in CRF patients, preferably the pre-dialysis stage, to achieve the best results. Longer studies will clarify the sustainability of the anti-body responses obtained.
Acknowledgment
The authors graciously acknowledge the support extended by Novartis, India and Panacea Biotech for providing GM-CSF (Leucomax) and recombinant HB vaccine (Enivac HB) respectively.
References
- Thomas P., Kirbuakaran M.G., Jacob C.K., Srinivasa N.S. Hepatitis B infection in a dialysis unit in South India. J. Assoc. Phys. Ind. 1987; 35: 284–285
- Malhotra K.K., Prabhakar S., Sharma R.K., Dash S.C., Singh R.N. Hepatitis B in a hemodialysis unit in New Delhi. J. Assoc. Phys. Ind. 1985; 33(3)216–217
- Mani M.K., Bhaskaran S., Prakash S.C. A study of hepatitis B surface antigen positive patients on hemodialysis and following transplantation. J. Assoc. Phys. Ind. 1994; 42: 363–365
- Krishnamurthy G., Kher V., Naik S. Defective lymphoproliferative responses and interleukin-2 production in chronic renal failure patients. Ind. J. Med. Res. 1995; 281–286
- Walz G., Kunzendorf U., Josimovic-Alasevico. Soluble interleukin-2 receptor and tissue polypeptide antigen serum concentrations in end stage renal failure. Nephron 1990; 56: 157
- Stachowski J., Pollok M., Barth C., Maciejewski J., Baldamus C.A. Non responsiveness to hepatitis B vaccination in haemodialysis patients: association with impaired TCR/CD3 antigen receptor expression regulating co-stimulatory processes in antigen presentation and recognition. Nephrol. Dial. Transplant 1994; 9: 144–152
- Opperman M., Kurts C., Zierz R. Elevated plasma levels of the immunosuppressive complement fragment Ba in renal failure. Kidney Int. 1991; 40: 939
- Girndt M., Sester M., Sester U., Kaul H., Kohler H. Defective expression of B7-2(CD86) on monocytes of dialysis patients correlates to the uremia associated immune defect. Kidney Int. 2001; 59(4)1382–1389
- Stevens C.E., Alter H.J., Taylor P.E., Zange E.A. Hepatitis B vaccine in patients receiving hemodialysis—Immunogenicity and efficacy. N. Engl. J. Med. 1984; 311: 496–501
- Fabrizi F., Di Filippo S., Marcelli D., Guarnori I. Recombinant hepatitis B vaccine use in chronic hemodialysis patients—Long term evaluation and cost effectiveness analysis. Nephron 1996; 72: 536–543
- Krishnamurthy G., Kher V., Naik S. Immunogenicity and efficacy of hepatitis B vaccination in Indian chronic renal failure patients on hemodialysis and after renal transplantation. Nephron 1996; 74: 424–425
- Jungers P., Devillier P., Salomon H. Randomized placebo controlled trial of recombinant interleukin-2 in chronic uremic patients who are non-responders to hepatitis B vaccine. Lancet 1994; 334: 856–857
- Donati D., Gastaldi L. Controlled trial of thymopentin in hemodialysis patients who fail to respond to hepatitis B vaccination. Nephron 1988; 50: 133–136
- Goldwater P.N. Randomized comparative trial of interferon-α vs. placebo in hepatitis B vaccine non-responders and hyporesponders. Vaccine 1994; 12: 410–414
- Senneseal J.J., Niepen P.V.D., Verbeelen D.L. Treatment with recombinant human erythropoietin increases antibody titers after hepatitis B vaccination in dialysis patients. Kidney Int. 1991; 40: 121–128
- Kapoor D., Aggarwal S.R., Singh N.P., Thakur V., Sarin S.K. Granulocyte macrophage colony stimulating factor enhances the efficacy of hepatitis B vaccine in previously unvaccinated hemodialysis patients. J. Viral. Hep. 1996; 6: 405–409
- Fabrizi F., Andruui S., Bacchini G., Corti M., Locatelli F. Intrademal vs. intramuscular hepatitis B re-vaccination in non-responsive chronic dialysis patients: a prospective randomized study with cost-effectiveness evaluation. Nephrol. Dial. Transplant 1997; 12: 1204–1211
- Ruef C., Coleman D.L. Granulocyte–macrophage colony stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev. Infect. Dis. 1990; 12: 41–62
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte–macrophage colony stimulating factor plus interluekin-4 and down regulated by tumor necrosis factor-α. J. Exp. Med. 1994; 179: 1109–1118
- Fischer H.G., Frosch S., Riske K., Reske-Kunz A.B. Granulocyte–macrophage colony stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J. Immunol. 1988; 141: 3882–3888
- Hess G., Kreiter F., Kosters W., Deusch K. The effect of granulocyte–macrophage colony stimulating factor (GM-CSF) on hepatitis B vaccination in hemodialysis patients. J. Viral. Hep. 1996; 3: 149–153
- Anandh U., Dhanraj P., Nayyar V., Ballal H.S. Granulocyte macrophage colony stimulating factor (GM-CSF) as an adjuvant to hepatitis B vaccination in hemodialysis patients: a preliminary report. Ind. J. Nephrol. 1997; 7: 108–111
- Sudhagar K., Chandrasekhar S., Rao M.S., Ravichandran R. Effect of granulocyte macrophage colony stimulating factor on hepatitis B vaccine in hemodialysis patients. J. Assoc. Phys. Ind. 1999; 47: 602–604
- Malhotra K.K., Saxena S., Mahajan M., Mishra P. Role of granulocyte macrophage colony stimulating factor in hepatitis B vaccination in hemodialysis patients—a prospective trial. Ind. J. Nephrol. 1999; 9: 38–40
- Anandh U., Bastani B., Ballal S. Granulocyte macrophage colony stimulating factor as an adjuvant to hepatitis B vaccination in maintenance hemodialysis patients. Am. J. Nephrol. 2000; 20: 53–56
- Evans T.G., Schiff M., Graves B., Agosti J., Baritt M.L. The safety and efficacy of GM-CSF as an adjuvant in hepatitis vaccination of chronic hemodialysis patients who have failed primary vaccination. Clin. Nephrol. 2000; 54(2)138–142
- Kher V., Aggarwal M., Bansal R. Potentiation of seroconversion to hepatitis B vaccination with GM-CSF in CRF. Abstracts of XXXth Annual Conference of Indian Society of Nephrology. PatnaIndia 1999
- Bommer J., Grussendorf M., Jilg W., Dienharndt F. Vaccination against hepatitis B in patients with renal insufficiency. Proc. Eur. Dial. Transplant Assoc. Eur. Ren. Assoc. 1985; 21: 300–305
- Dukes C.S., Street S.C., Starling J.F., Hamilton J.D. Hepatitis B vaccination and booster in predialysis patients: a 4-year analysis. Vaccine 1993; 11(12)1229–1232