Abstract
Background. Patients undergoing chronic hemodialysis have an increased risk of acquiring hepatitis B infection. Only 43–66% of dialysis patients develop effective anti-HBs titers after vaccination. Aim. To evaluate the effect of recombinant erythropoietin (rEPO) therapy and basal hemoglobin levels on the outcome of the immune response to four doses of IM 40 µg Engerix-B vaccination in hemodialysis and chronic kidney disease (CKD) patients before starting replacement therapy. Subjects and methods. One hundred and three patients were included in the study: 34 hemodialysis patients treated with rEPO (Group A), 36 predialytic patients who did not treated with rEPO (Group B) and 33 predialytic patients treated with rEPO (Group C). Plasma creatinine in predialytic patients was 2–7 mg/dL. All patients' HBsAg and anti-HBs antibodies were negative. Patients were immunized with IM 40 µg Engerix-B at 0, 1, 3, and 6 months. Anti-HBs titers were measured at 7th month. Results. Eighty seven point one percent of patients from group C developed protective anti-HBs titers compared with 69.4% from group B and 44.1% from group A (p = 0.001). Patients from all groups with baseline hemoglobin levels above 11 gr/dL developed protective anti-HBs titers significantly more than patients with baseline hemoglobin levels below 11 gr/dL (p<0.05). Conclusion. Predialytic patients treated with rEPO and with hemoglobin levels higher than 11 gr/dL had significantly better immune response outcomes to Engerix-B vaccination. Immunization against hepatitis B infection should be considered at early stages of CKD prior to the deterioration in kidney functions and the development of renal anemia.
Introduction
Patients undergoing chronic hemodialysis have an increased risk of acquiring hepatitis B virus infection due to defective immune response, resulting from T cell dysfunction.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]], Citation[[5]]
Seroconversion after hepatitis B vaccination has been estimated to occur when the level of anti-HBs is higher than 10 IU/l.Citation[[6]], Citation[[7]], Citation[[8]] Hepatitis B vaccination resulted in high antibody response in over 95% of healthy individuals.Citation[[9]] Immunogenicity in dialysis patients has been less satisfactory with poor vaccine response ranging from 43 to 66%.Citation[[9]], Citation[[10]], Citation[[11]], Citation[[12]], Citation[[13]] In order to improve the immune response in these patients several protocols had been examined including vaccination with intramuscular high doses,Citation[[14]] intradermal immunization,Citation[[15]] concomitant therapy with oral levamisole supplementation,Citation[[16]] alpha interferon,Citation[[17]] interleukin-2Citation[[18]] or granulocyte macrophage colony stimulating factor (GM-CSF).Citation[[19]] Although these protocols showed some improvement in the vaccination outcomes, the results were suboptimal. Only few protocols examined the efficacy of hepatitis B vaccination in chronic kidney disease (CKD) before starting renal replacement therapy (predialytic patients).Citation[[20]], Citation[[21]]
Erythropoietin (EPO) is a major determinant of the regulation of proliferation and differentiation of erythroid progenitor cells.Citation[[22]] Recombinant EPO (rEPO) is being used widely in the treatment of renal anemia more than one decade. Recent data indicate that rEPO have humoral and cellular immunomodulating properties.Citation[[23]], Citation[[24]], Citation[[25]]
The aim of the study was to evaluate the effect of rEPO therapy and basal hemoglobin levels on the outcome of the immune response to four doses of IM 40 µg Engerix-B vaccination in hemodialysis and chronic kidney disease (CKD) patients before starting replacement therapy.
Subjects and Methods
One hundred and three patients were included in the study: 34 hemodialysis patients who are on subcutaneous rEPO therapy: 80 units/kg in two divided doses weekly (Group A), 36 predialytic patients who had not been treated with rEPO (Group B) and 33 predialytic patients who are on rEPO therapy with the same schedule given to hemodialysis patients' (Group C). All patients HBsAg and anti-HBs antibodies were negative; their hemoglobin levels were below 12 gr/dL and without any evidence of iron, folic acid or vitamin B12 deficiencies. Plasma creatinine range of predialytic patients was 2–7 mg/dL and creatinine clearance test (CCT) was below 30 mL/min. Patients were immunized with IM 40 µg recombinant DNA hepatitis B vaccine (Engerix-B) at 0, 1, 3, and 6 months. The vaccine was applied in the deltoid muscle. Anti-HBs titers were measured at 7th month. Levels of antiHBs antibodies titers >10 IU/l were considered protective. The patients were not treated with blood transfusions or immunosuppressive drugs before and during the study and did not suffer from any active autoimmune disease. Basal plasma creatinine, complete blood count, albumin and CCT were examined again at 7th month. The baseline characteristics of the patients enrolled in the study are shown in .
Table 1. Baseline characteristics of the patients enrolled in the study
Statistical Analysis
Nominal variables: sex, type of kidney disease, and protective anti-Hbs titers were described by frequencies and percents. Quantitative variables: hemoglobin, serum creatinine, serum albumin, CCT, and age were described by mean and standard Wilcoxon rank sum test and Mann Whitney test were used to compare age, serum creatinine, serum albumin, and CCT between groups. Chi-square test was used to compare the development of protective anti-HBs titers between all groups. Fisher's exact test was used to compare the development of protective anti-HBs titers between patients with baseline hemoglobin levels higher and below 11 gr/dL.
Results
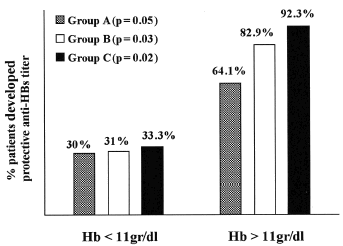
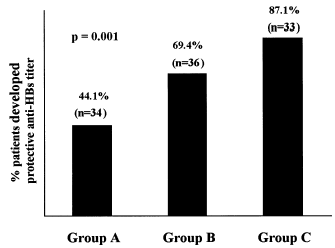
At 7th month 87.1% patients from group C developed protective anti-HBs titers compared with 69.4% from group B and 44.1% patients from group A (p = 0.001) (). Furthermore, 92.3% of predialytic patients with hemoglobin levels higher than 11 gr/dL who had been on rEPO therapy, developed protective anti-HBs titers compared to 33.3% with hemoglobin levels below 11 gr/dL (p = 0.02) ().
Figure 1. Development of protective anti-HBs titers in hemodialysis patients (Group A), predialytic patients who had not been treated with rEPO (Group B) and predialytic patients who are being on rEPO therapy (Group C).
Eighty two point nine percent of predialytic patients with hemoglobin levels higher than 11 gr/dL who did not receive rEPO therapy, developed protective anti-HBs titers compared with 31.0% with hemoglobin levels below 11 gr/dL (p = 0.03) (). Sixty four point one percent of hemodialysis patients with hemoglobin levels higher than 11 gr/dL who had been on rEPO therapy developed protective anti-HBs titers compared to 30.0% with hemoglobin levels below 11 gr/dL (p = 0.05) (). In all groups, no significant correlations were found between age, sex, type of kidney disease, serum creatinine, serum albumin, and the development protective anti-HBs titers.
Discussion
Only 43–66% of dialysis patients develop effective anti-HBs titers after vaccinationCitation[[9]], Citation[[10]], Citation[[11]], Citation[[12]], Citation[[13]] compared with over 95% of healthy individuals.Citation[[9]] All protocols used to improve the outcome of vaccination against hepatitis B infection in chronic dialysis patients were sub-optimal.Citation[[14]], Citation[[15]], Citation[[16]], Citation[[17]], Citation[[18]], Citation[[19]] This reduced immune response was related to defective immune response in uremic patients.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]], Citation[[5]] In our study, only 44.1% of hemodialysis patients developed protective anti-HBs titers ().
In order to improve the immunogenicity in dialysis patients, few protocols examined the efficacy of hepatitis B vaccination in patients suffering from CKD before starting renal replacement therapy.Citation[[20]], Citation[[21]] In one study, Carol et al. showed that immunization of predialytic patients with three double vaccine doses at 0, 1, and 6 months resulted in developing protective anti-HBs titers at 7th months in 60% of these patients.Citation[[20]] Our results showed that immunization of predialytic patients with four double vaccine doses at 0, 1, 3, and 6 months resulted in developing protective anti-HBs titers at 7th months in 87.1% of patients who had been on rEPO therapy compared with 69.4% of patients who did not receive rEPO therapy (p = 0.001), (). These results suggest that immunization of predialytic patients improve significantly the immune response to Engerix-B compared to hemodialysis patients (p = 0.001) (). Moreover, rEPO therapy improved significantly the immune response to Engerix-B in predialytic patients who had been on rEPO therapy (p = 0.001) ().
Our results suggest that in hemodialysis and predialytic patients with hemoglobin levels higher than 11 gr/dL, the immune response to Engerix-B was significantly better compared to patients with hemoglobin levels below 11 gr/dL (). To our knowledge, this association between the hemoglobin levels and the development of protective anti-HBs titer after hepatitis B vaccination was not reported before in patients suffering from CKD. Possible reasons might be a better general state of health in patients with higher hemoglobin, higher EPO levels or less level of inflammation, which is well known to be present in renal failure and to induce anemia. Recent data indicate that rEPO have humoral and cellular immunomodulating properties.Citation[[23]], Citation[[24]], Citation[[25]] rEPO increases immunoglobulins production and increase proliferation of human B cells and B cell lines.Citation[[23]] rEPO increases significantly CD4 and CD8 cells without changing significantly the CD4/CD8 ratio, decreases significantly the number of natural killer cells and improves the impaired phagocytic activity in hemodialysis patients.Citation[[24]] rEPO increases significantly in vitro T-cell mitogenic proliferation in hemodialysis patients.Citation[[25]] We suppose that rEPO may affect the immune response to Engerix-B through, at least, some of these immunomodulating properties.
There is general consensus that it is difficult to achieve protective anti-HBs titers following hepatitis B vaccination in patients suffering from CKD. Our results showed that the immune response to vaccination with four doses of IM 40 µg Engerix-B B in hemodialysis patients is still poor. On the other hand, vaccination against hepatitis B infection in predialytic patients treated with rEPO and where hemoglobin levels were higher than 11 gr/dL achieved better immune response to Engerix-B, but still slightly below the outcomes achieved in healthy individuals. Hemoglobin levels higher than 11 gr/dL were associated with better immune response outcomes. It may be that the hemoglobin level itself is not the major factor contributing to the improvement in immune response outcome, but it may be that hemoglobin levels higher than 11 gr/dL indicate higher EPO levels, less inflammation and better immunogenicity state. Therefore, successful correction of anemia in predialytic patients with rEPO, prior to vaccination against hepatitis B infection, can be associated with an improvement in the immunogenicity and play an important role in the outcome of immunization of CKD patients. In the light of our results, we conclude that vaccination against hepatitis B infection should be done at early stages of CKD prior to the deterioration in kidney functions and the development of renal anemia.
References
- Haubitz M., Ehlerding G., Beigel A., Heuer U., Hemmerling A.E., Thoma H.A. Clinical experience with a new recombinant hepatitis-B vaccine in previous non-responders with chronic renal insufficiency. Clin. Nephrol. 1996; 45: 180–182
- Kunori T., Fehrman I., Ringden O., Moller E. In vitro characterization of immunological responsiveness of uremic patients. Nephron 1980; 26: 234–239
- Raska K., Jr., Raskova J., Shea S.M., Frankel R.M., Wood R.H., Lifter J., Ghorial I., Eisinger R.P., Homer L. T cell subsets and cellular immunity in end-stage renal disease. Am. J. Med. 1983; 75: 734–740
- Chatenoud L., Dugas B., Beaurain G., Touam M., Drueke T., Vasquez A., Galanaud P., Bach J.F., Delfraissy J.F. Presence of preactivated T cells in hemodialyzed patients: their possible role in altered immunity. Proc. Nath. Acad. Sci. USA 1986; 83: 7457–7461
- Rubin L.A., Kurman C.C., Fritz M.E., Biddison W.E., Boutin B., Yarchoan R., Nelson D.L. Soluble interleukin-2 receptors are released from activated human lymphoid cells in vitro. J. Immunol. 1985; 135: 3172–3177
- Seaworth B., Drucker J., Starling J., Drucker R., Stevens C., Hamilton J. Hepatitis B vaccine in patients with chronic renal failure before dialysis. J. Infect. Dis. 1988; 157: 332–337
- Hadler S., Francis D., Maynard J., Thompson S.E., Judson F.N., Echenberg D.F., Ostrow D.G., Mally P.M., Penley K.A., Altman N.L. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N. Engl. J. Med. 1986; 315: 209–214
- Eddleston A. Modern vaccines: hepatitis. Lancet 1990; 335: 1142–1145
- Szmuness W., Stevens C.E., Harley E.J., Zang E.A., Oleszko W.R., William D.C., Sadovsky R., Morrison J.M., Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N. Engl. J. Med. 1980; 303: 833–841
- Stevens O.E., Szmuness W., Goodman A.I., Weseley S.A., Fotino M. Hepatitis B vaccine immune response in hemodialysis patients. Lancet 1980; 11: 1211–1213
- Pasko M.R., Bartholomew W.R., Beam T.R., Amsterdam D., Cunningham E.E. Long-term evaluation of the hepatitis B vaccine (Heptavax-B) in hemodialysis patients. Am. J. Kidney Dis. 1988; 11: 326–331
- Jilg W., Schmidt M., Weinel B., Kuttler T., Brass H., Bromer J., Muller R., Schulte B., Schwarzbeck A., Deinhardt F. Immunogenicity of recombinant hepatitis B vaccine in dialysis patients. J. Hepatol. 1986; 3: 1900–1905
- Fernandez E., Betriu M.A., Gomez R., Montoliu J. Response to the hepatitis B virus vaccine in hemodialysis patients: influence of malnutrition and is importance as a risk factor for morbidity and mortality. Nephrol. Dial. Transplant. 1996; 12: 623–624
- Navarro J.F., Teruel J.L., Mateos M.L., Marcen R., Ortuno J. Antibody level after hepatitis B vaccination in hemodialysis patients: influence of hepatitis C virus infection. Am. J. Nephrol. 1996; 16: 1695–1697
- Mettang T., Schenk U., Thomas S., Machleidt C., Kiefer T., Fisher F.P., Kuhlmann U. Low-dose intradermal versus intramuscular hepatitis B vaccination in patients with end-stage renal failure. A preliminary study. Nephron. 1996; 72: 192–196
- Ayli M.D., Ensari C., Ayli M., Mandioglu Mut S. Effect of levamidasole supplementation to hepatitis B vaccination on the rate of immune response in chronic hemodialysis patients. Nephron 2000; 84: 291–292
- Ozener C., Fak A.S., Avsar E., Cinar S., Lawrence R., Akoglu E. The effect of alpha interferon therapy and short-interval intradermal administration on response to hepatitis B vaccine in haemodialysis patients. Nephrol. Dial. Transplant. 1999; 14: 1339–1340
- Mauri J.M., Valles M. Effect of recombinant interleukin-2 and revaccination for hepatitis B in previously vaccinated, non-responer, chronic uraemic patients. Nephrol. Dial. Transplant. 1997; 12: 729–732
- Anandh U., Bastani B., Ballal S. Granulocyte-macrophage colony-stimulating factor as an adjuvant to hepatitis B vaccination in maintenance hemodialysis patients. Am. J. Nephrol. 2000; 20: 53–56
- Carol S.D., Alan C.S., James F.S., Hamilton J.D. Hepatitis B vaccination and booster in predialysis patients: a 4-years analysis. Vaccine 1993; 11: 1229–1232
- Fraser G.M., Ohana N., Fenyves D., Neumann L., Chazan R., Niv Y., Niv Y., Chaimovitch S. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J. Hepatol. 1994; 21: 450–454
- Krantz S.B. Erythropoietin. Blood 1991; 77: 419–434
- Kimata H., Yoshida A., Ishioka C., Masuda S., Sasaki R., Mikana H. Human recombinant erythropoietin directly stimulates B cell immunoglobulin production and proliferation in serum free medium. Exp. Immunol. 1991; 85: 151–156
- Huraib S., Abu Aisha H., Al Momen A., Al Wakeel J., Memon N., Al Tuwaijri A. Effect of recombinant human erythropoietin on lymphocyte phenotyping and phagocyte activity in hemodialysis patients. Am. J. Kidney Dis. 1997; 29: 866–870
- Shurtz Swirski R., Kristal B., Shkolnik T., Weissman I., Shapiro G., Shasha S.M. Short-term effect of erythropoietin on T-cell mitogenic proliferation in chronic renal failure patients. Nephron 1996; 72: 27–29