Abstract
A structural defect of the non-vascular component of a nephron namely vesicoureteric reflex (VUR) can induce injury to the vascular component, which is reflected by the alteration in intrarenal hemodynamics. A mild alteration in intrarenal hemodynamics was observed in grades I–II VUR which revealed (a) mild reductions in renal plasma flow (RPF) 543 ± 104 mL/min/1.73 m2; in peritubular capillary flow (PTCF) 438 ± 103 mL/min/1.73 m2; in glomerular filtration rate (GFR) 105 ± 19 mL/min/1.73 m2 and in ultrafiltration coefficient (KFG) 0.04 ± 0.01 mL/s/mmHg; (b) normal values of filtration fraction (FF) 0.2 ± 0.04, of intraglomerular hydrostatic pressure (PG) 50 ± 0.3 mmHg, and of afferent arteriolar resistance (RA) 2261 ± 718 dyne s cm−5; and (c) a slight elevation of efferent arteriolar resistance (RE) 3914 ± 962 dyne s cm−5. In contrast, a moderately severe alteration in intrarenal hemodynamics was observed in severe VUR (grades III up) which revealed greater reductions in RPF 267 ± 114 mL/min/1.73 m2, in PTCF 195 ± 90 mL/min/1.73 m2, in GFR 72 ± 34 mL/min/1.73 m2 and in KFG 0.03 ± 0.01 mL/s/mmHg; and elevation of PG 53 ± 2 mmHg, of filtration fraction 0.27 ± 0.07, of RA 4557 ± 2340 dyne s cm−5 and of RE 9417 ± 4163 dyne s cm−5. Such an alteration in intrarenal hemodynamics observed in severe VUR induces both intraglomerular hypertension (elevated PG) and an exaggeratedly reduced PTCF. This intrarenal hemodynamic defect is due to the glomerular endothelial dysfunction and its hemodynamic maladjustment. In accordance with the preceding information, treatment to correct the hemodynamic maladjustment is likely to improve renal function and prevent renal disease progression.
Introduction
A nephronal structure consists of Citation[[1]] a vascular component such as glomerular capillary and postglomerular microcirculation which from the functional and structural point of view is closely contact withCitation[[2]] the non-vascular component namely tubulointerstitium and tubular luminal space. Any disorder triggering on one component (vascular) would likely affect another component (non-vascular) and vice versa. In respect to this vision, an injury to the glomerular endothelial cell (vascular) with a simultaneous dysfunction of the glomerular endothelium associated with a hemodynamic alteration has been delineated in the early course of mild glomerulonephropathy such as mesangial proliferative nephrosis and acute poststreptococcal glomerulonephritis.Citation[[1]], Citation[[2]] It is characterized by (1) the expression of procoagulant activity namely the shortened platelet half-life, fibrin deposit in the kidney and an elevated level of fibrin degradation product in the urine and serumCitation[[3]], Citation[[4]] (2) the defect in releasing vasodilator which is reflected by the reduction in renal plasma flow determined by the intrarenal hemodynamic study.Citation[[5]] In contrast to this mild form, in severe form namely nephrosis with focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis and chronic glomerulonephritides, such an injury to the vascular component would also affect the non-vascular component (tubulointerstitium) and induce tubulointerstitial fibrosis.Citation[[6]] A cause-and-effect relationship between the vascular (hemodynamics) and the involvement of non-vascular (tubulointerstitial fibrosis) has recently been illustrated in such a way that (1) there is an inverse correlation between the renal perfusion and the magnitude of tubulointerstitial fibrosis and (2) the reduction in renal perfusion precedes the development of tubulointerstitial fibrosis.Citation[[7]]
In accordance with the preceding viewpoint, it is interesting to know whether a disorder initially triggering the non-vascular component such as the congenital anomaly associated with vesicoureteric reflux (VUR) would also affect the vascular component or the hemodynamic function of the nephron. An upstream elevation of intraureteral pressure during straining at micturition would affect the glomerular filtering process and thereby affect intrarenal hemodynamics. If so, is the altered intrarenal hemodynamics likely responsible for the renal parenchymal injury in the severe form so called reflux nephropathy?
Materials and Methods
Studies were conducted on 20 patients associated with 10 patients with mild form of VUR (grades I and II) and 10 patients with severe form of VUR (grades III–V). The intrarenal hemodynamic study was performed with a simultaneous determination of renal plasma flow (RPF) using 131I-labeled orthoiodohippuric acid (hippuran) and the glomerular filtration rate (GFR) using 99mTc-labeled diethylene triamine pentaacetic acid (DTPA) and the peritubular capillary flow (PTCF) which was determined by the substraction of GFR from RPF had been accomplished by the previously described method.Citation[[8]]
Results
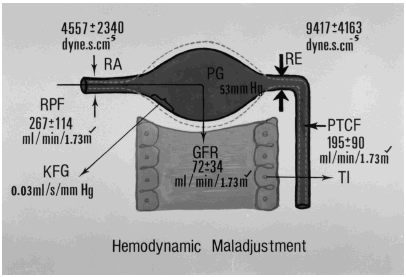
The results indicated that in mild form of VUR (grades I and II), there was a mild alteration in intrarenal hemodynamics which was characterized by (1) mild reductions in RPF (mean 547 ± 98 mL/min/1.73 m2), in PTCF (mean 438 ± 103 mL/min/1.73 m2), in GFR (mean 105 ± 19 mL/min/1.73 m2) and in ultrafiltration coefficient of glomerulus (KFG) mean 0.04 ± 0.01 mL/s/mmHg; (2) a normal filtration fraction (FF) mean 0.2 ± 0.04, a normal intraglomular hydrostatic pressure (PG) mean 50 ± 0.3 mmHg and a normal afferent arteriolar resistance (RA) mean 2261 ± 718 dyne s cm−5; (3) a mild elevation in efferent arteriolar resistance (RE) mean 3914 ± 962 dyne s cm−5. In contrast, in severe VUR (grades III to V), there were (1) moderate reductions in RPF (mean 267 ± 114 mL/min/1.73 m2), in PTCF (mean 195 ± 70 mL/min/1.73 m2), in GFR (mean 72 ± 34 mL/min/1.73 m2) and in KFG (mean 0.03 ± 0.01 mL/s/mmHg), (2) moderate elevations in FF (mean 0.27 ± 0.07), in PG (mean 53 ± 2 mmHg), in RE (mean 9417 ± 4163 dyne s cm−5), .
Table 1. Intrarenal hemodynamics in VUR
Discussion
A mild alteration in intrarenal hemodynamics observed in mild VUR is similar to that observed in mild form of glomerulonephropathy such as mesangial proliferative nephrosis or acute poststreptococcal glomerulonephritis.Citation[[5]] In contrast, a moderately severe intrarenal hemodynamic alteration has been observed in severe VUR—the type of VUR that is likely to be associated with renal parenchymal injury and renal disease progression. In this context, such an altered intrarenal hemodynamics is due to an inability of glomerular endothelium to release an adequate amount of endothelium-dependent vasodilators by which it induces a hemodynamic maladjustment with a preponderant constriction at the efferent arteriole (). The preferential constriction at the efferent arteriole in the presence of decreased ultrafiltration coefficient of the glomerulus would allow a more inflow of blood and a less outflow of blood and thereby induce 2 crucially important hemodynamic impacts. (1) Proximal to the constriction site at the efferent arteriole, it induces an elevation of intraglomerular hydrostatic pressure and intraglomerular hypertension. (2) Distal to the constriction site at the efferent arteriole, the preponderant constriction at the efferent arteriole would exaggeratedly reduce the peritubular capillary flow which supplies the tubulointerstitium. If this state of hemodynamic maladjustment were not corrected, it would likely induce a sustained ischemic injury to the tubulointerstitial structure. In this regard, an ischaemia or a low fluid shear stress state can induce endothelial cell dysfunction by expressing adhesion molecules, proinflammatory cytokines, growth factors namely transforming growth factor beta, platelet derived growth factor, reactive oxygen species and vasoconstrictors namely angiotensin II, endothelin, thromboxane A2 by which they would culminate in the inflammatory processes in the tubulointerstitium which is dependent upon the volume of PTCF.Citation[[9]] In addition, a superimposed infection in the urinary tract system commonly encountered in association with VUR would further exaggerate the inflammatory damage and eventually induce a progressive tubulointerstitial lesion so called the reflux nephropathy.
In accordance with the preceding information, a treatment to correct the hemodynamic maladjustment is likely to suppress or prevent the renal disease progression in association with VUR as it does occur in treating the hemodynamic maladjustment and preventing the renal disease progression in severe forms of glomerulonephropathy.Citation[[6]], Citation[[7]], Citation[[8]] A preliminary study addressed to this specific issue has been performed with vasodilators and yielded a promising result.Citation[[10]] An improvement in renal function has been observed following the correction of intrarenal hemodynamic maladjustment.
References
- Futrakul P. Kinetic evaluation of hemostasis in the steroid-sensitive nephrotic syndrome. J. Singapore Ped. Soc. 1977; 19: 273–275
- Futrakul P. Coagulation in glomerulonephritis and nephrotic syndrome: its therapeutic implication. Asian Manual of Nephrology, T. Takeuchi, N. Sugino, K. Ota. SEAMIC, Tokyo 1981; 89–96
- Futrakul P., Poshyachinda M., Mitrakul C. Focal sclerosing glomerulonephritis: a kinetic evaluation of hemostasis and the effect of anticoagulant therapy: a controlled study. Clin. Nephrol. 1978; 10: 180–186
- Stiehm E.R., Kuplic L.S., Nehling D.T. Urinary fibrin split products in human renal disease. J. Lab. Clin. Med. 1971; 77: 843–848
- Futrakul N., Futrakul P., Poshyachinda M. Intrarenal hemodynamic alteration in mesangial proliferative nephrotic syndrome with steroid resistance: effect of vasodilators. Nephron 1994; 66: 366–367
- Futrakul P., Pochanugool C., Poshyachinda M. Intrarenal hemodynamic abnormality in severe form of glomerulonephritis: therapeutic benefit with vasodilators. J. Med. Assoc. Thai. 1992; 75: 375–385
- Futrakul N., Yenrudi S., Sensirivatana R. Peritubular capillary flow determines tubulointerstitial disease in idiopathic nephrotic syndrome. Ren. Fail. 2000; 22: 329–335
- Futrakul P., Poshyachinda M., Futrakul N. Intrarenal hemodynamics alterations and tubular functions in nephrotic syndrome associated with focal segmental glomerulosclerosis (FSGS): a pathogenetic and therapeutic implicaiton. Current Therapy in Nephrology, V.E. Andreucci, A. Dal Canton. Milan, Wichtig 1993; 107–114
- Wei Z., Costa, Al-Medhi A.B., Dodia C., Muzykantor V., Fisher A.B. Simulated ischemia in flow-adapted endothelial cells lead to generation of reactive oxygen species and cell signaling. Circ. Res. 1999; 85: 682–689
- Futrakul N., Laohaphaibul A., Futrakul P., (unpublished data)