Abstract
A 34-year-old female with end-stage renal disease was admitted for severe metabolic acidosis, uremic encephalopathy, pericarditis and severe anemia following a bout of acute gastroenteritis. She improved on aggressive medical management including intensive hemodialysis and was initiated onto maintenance heparin-free hemodialysis (twelve hours per week) and discharged. After a week, she presented with fever with chills and rigors for three days, was toxic, severely orthopenic and had a pulsus paradoxus of 36 mmHg. Echocardiography suggested cardiac tamponade. Aspiration revealed frank pus with polymorphonuclear predominance and Staphylococcus aureus on culture. CT of the thorax revealed pericardial effusion. In the absence of any obvious septic foci, concomitant pleuro-pulmonary sepsis, mediastinal or intra-abdominal pathology; a diagnosis of “acute primary purulent pericarditis” was made. Patient was put on parenteral antibiotics-ceftriaxone and metrogyl. Vancomycin was added after sensitivity results. Pericardial drainage was required initially. After toxemia improved, paradox decreased and fever subsided, the pericardial catheter was removed and antibiotics continued for a period of four weeks. Maintenance hemodialysis was continued during hospital stay and after discharge.
Introduction
The course of a chronic renal failure patient is punctuated by a number of intercurrent illnesses. Uremic and dialysis associated pericarditis are well-known complications in end-stage renal disease patients. However, we report an unusual presentation of pericarditis—purulent pericarditis, in a chronic renal failure patient.
Report of A Case
A 34-year-old female diagnosed as a case of chronic renal failure (etiology chronic glomerulonephritis) was on regular follow-up at our Nephrology clinic for a year, on conservative medical management. She presented with a 5-day history of fever, loose motions and vomiting suggestive of acute gastroenteritis and altered sensorium for last 2 days. She was admitted to our institution with tachycardia, hypotension, metabolic acidosis, early uremic encephalopathy, and pericarditis (as evidenced by a pericardial rub). Patient responded to conservative medical management and heparin-free hemodialysis through a double-lumen femoral catheter. Echocardiography revealed minimal pericardial effusion with concentric left ventricular hypertrophy and diastolic dysfunction. The femoral hemodialysis catheter was removed at discharge and the catheter or the local site did not reveal any evidence of infection, although catheter tip was not sent for culture. The patient was advised for maintenance heparin-free dialysis (in view of pericarditis) on outpatient basis and planned for arteriovenous fistula. However, the patient failed to report for hemodialysis, and presented after 1 week with complaints of fever with chills and rigors and severe breathlessness for 3 days.
Patient was febrile (103.4°F), BP-180/90 mmHg with a paradox of 36 mmHg, pulse 112 bpm, regular, and patient was orthopenic (respiratory rate 36/min). Physical examination revealed pallor, anasarca, tender hepatomegaly, bilateral crepitus lower half of chest, heart sounds were distant; no murmur or pericardial rub or knock was present.
ECG showed sinus tachycardia with low voltage complexes and widespread ST-T changes. Chest X-ray revealed bilateral pleural effusion and moneybag appearance of the cardiac silhouette suggestive of pericardial effusion. Echocardiography suggested massive pericardial effusion with cardiac tamponade with right ventricular diastolic collapse, without any evidence of myocardial abscess or infective endocarditis. A pigtail catheter was inserted for pericardial drainage.
Hematological investigations revealed hemoglobin of 10 g/dL, leucocytosis (TLC—15,000/mm3, P78, L20, E2) and ESR of 50 mm at the end of 1st hour, blood urea, 180 mg/dL and serum creatinine, 4.2 mg/dL. Blood, urine, and sputum cultures were sterile. Pericardial fluid revealed proteins, 4.6 g/dL, sugar, 12 mg/dL, cells, 46,200/mm3, P97 L3, abundant pus cells, but no bacteria on Gram's stain and no acid-fast bacilli on Ziehl–Neelsen staining. Culture of pericardial fluid grew Staphylococcus aureus, sensitive to vancomycin, amikacin, and gentamicin. Pleural effusion was tapped which was transudative and culture was sterile.
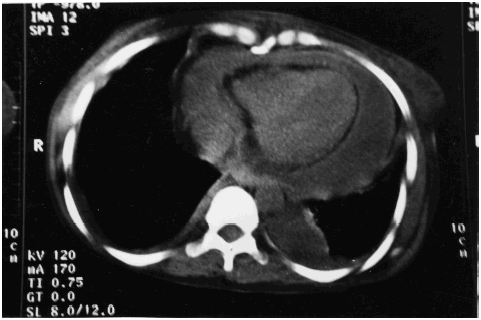
Non-contrast computerized tomography of the chest revealed hypodense collection in the pericardial cavity suggestive of pericardial effusion, with pericardial thickening and bilateral pleural effusion (). There was no evidence of mediastinal or pulmonary parenchymal pathology. No loculations/septae in the pericardial or pleural fluid were obvious. Ultrasonographic study of the abdomen did not reveal any sub-diaphragmatic or intra-abdominal or hepatic pathology or evidence of infection. A diagnosis of primary purulent (staphylococcal) pericarditis with end-stage renal disease was made.
Figure 1. Illustrates pericardial effusion with thickened pericardium and bilateral pleural effusion.
The patient was started on parenteral broad-spectrum antibiotic (ceftriaxone) with anaerobic cover (metronidazole). Vancomycin was added after the sensitivity report. She was re-initiated on maintenance heparin-free hemodialysis. Patient's symptoms improved, orthopnea settled and paradox decreased and the patient was afebrile after 5 days. Two hundred and fifty milliliters of frank pus was drained through the pigtail catheter at the time of insertion and another 250 mL drained over next 3 days. On the fifth day the drainage was minimal and keeping in view the clinical improvement as well, the catheter was removed on the sixth day. The treatment was continued for a total period of 4 weeks following which the patient was discharged with advise to continue maintenance heparin-free hemodialysis (twelve hours per week) at our center. Surgical intervention in this case could not be planned as the patient had refused to provide written consent besides having a poor prospect of renal transplantation due to financial constraints.
Discussion
Pericarditis envisages a response that may be an acute inflammation (dry pericarditis), effusion with or without pericarditis and fibrosis with or without constriction. Acute purulent pericarditis is a rare entity, predominantly seen in children and immunocompromised.Citation[[1]] In a series of 76 primary acute pericarditis patients (duration of illness>7 days) only two cases of purulent pericarditis were reported.Citation[[2]] Although reports on purulent pericarditis are available, the rarity of primary purulent pericarditis is striking. In fact, in a review on purulent pericarditis, primary pericarditis was not discussed.Citation[[1]] This is partly because diagnosis of purulent pericarditis is in itself difficult and many cases are detected on autopsy.Citation[[3]], Citation[[4]] Moreover, in primary pericarditis where there is no obvious source of infection, and the course being a malignant one, the diagnosis is elusive.
Purulent pericarditis is often an acute and catastrophic illness, although rare exceptions may occur.Citation[[5]] There are prominent fever and chills with rapid development of tamponade. In fact, it may present as tamponade itself. In the pre-antibiotic era, the main etiologic agents were pneumococcus (51%), staphylococcus (19%) and streptococcus (10%). However in the antibiotic era, Staphylococcus (22%) ranks as the foremost etiological agent,Citation[[6]], Citation[[7]] Gram-negative bacteria have shown a resurgence (32%), while pneumococcus (9%), being penicillin sensitive has shown a decline in incidence. In fact from a study of purulent pericarditis in the tropics, Staphylococcus aureus was reported in 50% cases as an etiological agent.Citation[[2]]
Pericardium is rarely a primary site of infection; the source may be a local or distant focus.Citation[[3]] It is to emphasize that primary pericarditis does not mean that the infection has directly been introduced into the pericardium. It is but obvious that the infection to the pericardium predominantly will reach either secondary to a septic focus somewhere else in the body or it is a result of transient bacteriemia resulting in seeding of the pericardium with pyogenic infection. The adjective “primary” in “primary pericarditis” simply connotes that the source of infection is elusive. Purulent pericarditis may occur by any of the three mechanismsCitation[[8]]:
Invasion from contiguous foci. Commonly, the lung in pneumonia, empyema, mediastinitis, myocardial abscess, infective endocarditis, subdiaphragmatic abscess and rarely, odontogenic/peritonsillar abscess tracking down facial planes or post-appendicitis/pancreatitis.
Traumatic implantation. Cardiothoracic surgery, wound infection, trauma.
Hematogenous spread. Rarely, pre-existing non-bacterial pericardial effusion as in rheumatoid arthritis, lupus erythematosus, sarcoid or uremia is infected hematogenously. When this type of pericarditis occurs without any concomitant evident infective focus; it is termed as “primary bacterial pericarditis”.
The course of illness in renal failure patients is punctuated by many intercurrent illnesses, of which infections are major contributors in tropical countries like ours. Although these patients commonly have uremic pericarditis and dialysis-associated pericarditis, purulent pericarditis is rare. To the best of our knowledge, occasional case reports exist of staphylococcal pericarditis in renal failure patients.Citation[[9]] The present case is of primary staphylococcal pericarditis, which is an extremely rare entity, in an end-stage renal disease patient. This case had a uremic pericarditis and presented after 7 days in a toxic state with high fever and cardiac tamponade. It is postulated that a trivial bacteriemia may result in seeding of the sterile pericardial effusion resulting in purulent pericarditis. The source of bacteriemia in this case may have been the femoral dialysis catheter or intravenous cannula that the patient was harboring earlier. Staphylococcus aureus is the leading cause of hemodialysis catheter related bloodstream infections, contributing 33–80% of the organisms cultured from blood samples.Citation[[10]] Asymptomatic catheter-related colonization occurs in 10–55% of hemodialysis catheters.Citation[[10]] However, since the local site of cannula was healthy at second admission and there was no local inflammation at the time of its removal, we cannot hold the dialysis catheter as a culprit for bacteriemia; more so in the presence of sterile blood cultures. Nevertheless, there is every likelihood of a transient bacteriemia since the patient earlier harbored a dialysis-catheter, an intravenous access and had received multiple injections and intensive dialysis sittings. It is probable that the patient had a bacteriemia during the first hospital stay that seeded the pericardium and resulted in her readmission after a week of discharge. In the absence of any other obvious infective foci, primary staphylococcal pyopericardium was diagnosed and is being discussed because of its rarity. The possibility that nonsuppurative pericardial disease may predispose to purulent disease was suggested way back in 1942 and reemphasized in 1975.Citation[[3]] It is believed that the rich fibrinous exudates and pericardial fluid provide fertile culture medium for bacterial colonization, particularly in debilitated and immunocompromised patients.
Management of purulent pericarditis includes systemic antibiotics and pericardial drainage and exploration. Antibiotics have a good penetration into the pericardial fluid and the most specific anti-microbial should be given for 3–4 weeks.Citation[[1]] Penicillins or cephalosporins are the drugs of choice, vancomycin may be added for methicillin-resistant S. aureus and aminoglycosides added if patient is post-cardiac surgery, has genito-urinary sepsis or is immunocompromised. Recent recommendations suggest vancomycin use to be restricted to methicillin resistant Staphylococcus aureus, although resistant to vancomycin is also being reported of late.Citation[[10]] Pericardial drainage is mandatory because infections tend to loculate resulting in adhesions and constriction. Surgical drainage is preferred over percutaneous drainage as it permits pericardiectomy in case of constriction. Failure to improve after therapy is an important clinical clue to loculation and potential constriction. In critical tamponade, simple drainage followed by exploration is recommended. Our patient responded to simple percutaneous drainage. Patient's refusal to undergo surgery prevented over-zealous attempts at pericardiectomy.
Mortality is high and ranges in between 25 and 75%, 45% have been reported even with appropriate therapy.Citation[[5]] Poor prognostic factors include delay in recognition, absence of early surgical drainage, presence of cardiac tamponade, myocardial involvement, age of patient, Staphylococcus aureus as etiologic agent. Despite presence of several poor prognostic factors and co-morbid illness, our patient had a successful outcome.
References
- Hall I.P. Purulent pericarditis. Postgrad. Med. J. 1989; 65: 444–448
- Permanyer-Miralda G., Sagrista-Sauleda J., Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 patients. Am. J. Cardiol. 1985; 56: 623–630
- Corachan M., Poore P., Hadley G.P., Tanner A. Purulent pericarditis in Papua New Guinea: report of 12 cases and review of the literature in a tropical environment. Transactions of the Royal Society of Tropical Medicine and Hygiene 1983; 77: 341–343
- Klacksmann P.G., Bulkley B.H., Hutchins G.M. The changed spectrum of purulent pericarditis. An 86 year autopsy experience in 200 patients. Am. J. Med. 1977; 63: 666–673
- Tsai J., Shands J.W. Staphylococcal pericarditis. An atypical presentation. Arch. Intern. Med. 1989; 149: 953–954
- Brook I., Frazier E.H. Microbiology of acute purulent pericarditis. A 12 year experience in a military hospital. Arch. Intern. Med. 1996; 156: 1857–1860
- Jayashree M., Singhi S.C., Singh R.S., Singh M. Purulent pericarditis: clinical profile and outcome following surgical drainage and intensive care in children in Chandigarh. Ann. Trop. Pediatr. 1999; 19: 377–381
- Spodick D.H. Pericardial diseases. Heart Disease, 6th Ed., E. Braunwald, D.P. Zipes, P. Libby. WB Saunders Co., Philadelphia 2001; 1823–1876
- Mako J., Jansen J., Bognar B., Farago A. Purulent pericarditis caused by Staphylococcus aureus in two patients undergoing haemodialysis. Int. Urol. Nephrol. 1985; 17: 79–83
- Mermel L.A., Farr B.M., Sherertz R.J., Raad I.I., O'Grady N., Harris J.S., Craven D.E. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 2001; 32: 1249–1272
