Abstract
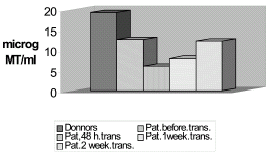
The plasma metallothionein concentration was studied in renal transplant patients. These patients are submitted to an attack of free radicals catalyzed by metals such as copper, zinc, cadmium, and others. The function of metallothionein is to bind toxic metals inhibiting the attack of free radicals and oxidative stress that patients receiving renal transplants are submitted to. This is the reason for studying this protein in this work. The metalloprotein was separated from the plasma by thermoprecipitation and molecular exclusion chromatography with Sephadex G-75 followed by anionic-ionic exchange chromatographic purification with a CINa gradient. Identification was done by SDS electrophoresis in acrylamide gel with markers and commercial protein. Finally, determinations were made by atomic absorption, silver saturation method. In this work, determinations were made in the plasma of 11 patients before and 48 h and 1 and 2 weeks after renal transplantation. The same study was carried out in parallel in a control group of 11 blood donors. The results obtained show the existence in the plasma of metallothionein, with lower concentrations in patients than in controls (19 ± 1.2 mg vs. 12 ± 1.2 mg). The levels were lowest in the patient group analyzed 48 h after having received the transplant (6.34 mg) and had recovered slightly one and two weeks later.
Introduction
Metallothionein is a protein rich in cysteine, with a content of 25%, and a molecular weight of 6000–9000 daltons. It is capable of binding heavy metals giving it a detoxifying action in the organism.Citation[[1]] Levels of this protein have been studied in organs but only a few works have analyzed levels in plasma.Citation[[2]] The metals that bind to this protein also induce its synthesis and the most relevant of these are silver, bismuth, cadmium, copper, zinc, and mercury.Citation[[3]] The kidney is the organ most affected by the toxicity of these metals, especially by cadmium that acts on the cells of the glomeruli.Citation[[4]] The metallothionein concentration in the kidney is around 394 mg per g and is expressed in epithelial cells of the renal glomeruli.Citation[[5]] In the process of renal transplantation, oxidative stress has been observedCitation[[6]], Citation[[7]] as a consequence of the attack of free radicals due to the presence of the metals. Also, low values of glutathione and antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase in addition to renal function index are observed. There is also a rise in lipid peroxidation during transplantation processes.Citation[[7]]
Therefore, in the light of these findings, metallothionein could play an antioxidant role in renal transplantation because of its ability to bind toxic metals thus preventing the formation of free radicals.
Material and Methods
For the isolation of metallothionein, the plasma of 11 patients was used before renal transplantation and at different times after transplantation. For a comparison, the same experiment was also carried out using 11 blood donors as controls. The protein was isolated by using the following procedure: 1. Thermoprecipitation at −100°C and at +100°C. Afterwards, the proteins precipitated at these temperatures were separated off and the metallothionein in solution was passed through an exclusion column with Sephadex G-75. The fractions eluted and collected in a collector, corresponding to the absorption peak, (), were concentrated by lyophilization after which the protein was purified by anionic-exchange chromatography with DEAE Sephadex A-25 and a gradient of CINa (). Protein determinations were done using atomic absorption on the basis that 1 mol of this protein binds 17 mol of silver, by the so-called Ag-saturation method ().
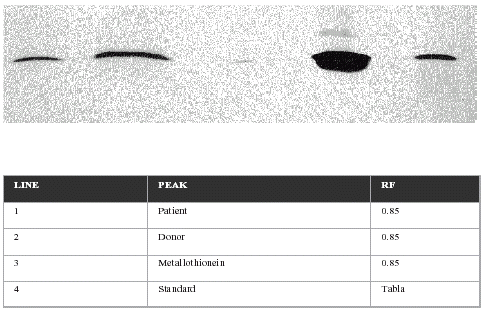
Previously, metalloprotein was identified by electrophoresis in SDS acrylamide gel with commercial peptides and crude protein of known molecular weight. The protein obtained emigrated as a single band at the height of commercial preparations ().
Results
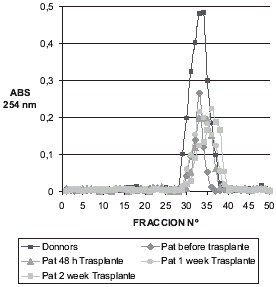
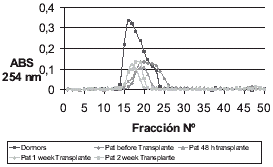
The results obtained from this study are shown in and . shows a molecular exclusion chromatogram of patients and controls. A peak in absorbance can be observed of 0.48 nm for controls read at 254 nm that is much higher than that obtained for patients before transplantation, 48 h after transplantation, one week after transplantation, and two weeks after that, present absorption peaks between 0.25 and 0.18, respectively. shows the ion-exchange chromatogram with superimposed curves. The values observed are lower owing to purification of the protein. The chromatogram of the control group also presents a low peak of 0.35 nm and transplanted patients present one of 0.12 nm.
Determination of metallothionein by the silver saturation method is shown in . As can be observed, the results obtained by these three methods are reproduced perfectly. Lower values are obtained in patients than in controls and lower values after transplantation, especially at 48 h that recover two weeks after. Finally, SDS electrophoresis results are presented in . The first line shows metallothionein levels in patients, the second in the control group, the third corresponds to commercial metallothionein and the fourth to proteins with a similar molecular weight to metallothionein.
Discussion
It has been studied that the Metallothionein concentrations in plasma transplant patients before and after transplantation, at different times of it, the evaluation was going out by means of isolation exclusion molecular chromatography with Sephadex G-75. The results obtained showed only a peak in the chromatogram of 0.2 absorbancy which a maximum about fraction n° 30 while in the control group it was at 0.5 absorbancy in the same fraction. These results showed that the all protein excluded are higher in donors than in patients and these values decreases after transplant (). One property of Metallothionein is also to regulate the homeostasis of zinc and copper. These metals induce the Metallothionein synthesis by activating the gene, which codifies the synthesis of Metallothionein.
Nevertheless, during transplant the zinc concentration decreases in these patients. Purification was made by ion exchange chromatography in gradient of NaCl going from 100 mM to 0.5 M concentrations, in Sephadex A-25 with this step the absorbancy in a transplant is reduced also to respect control group founding a pick at 0.15 nm against control group of 0.3 () this results are according with the obtained by Garcia Chillon in plasma patients in dialysis.Citation[[2]] Identification of Met was performed on SDS electrophoresis in acrylamide with two gel at different concentration, in this step the protein were identified by comparison with a commercial Met and know molecular weigh peptides, shown the existence of this protein in the plasma () although its concentration is less than in renal cortex and liver.
Finally the Silver saturation methodCitation[[8]] give to us the really concentration of purified protein () where you can see levels of Metallothionein with decrement of this protein respect the control group and also it is shown also how the protein decrease along transplant time, especially during 48h after and a weak, due probably to free radical explosion, but is going to recouped to the second weak finally show the protein existence in plasma by SDS electrophoresis which emigration band is at the same level that the pure commercial Metallothionein. The results obtained in this work look like this protein could be a renal marker in transplant.
References
- Kagi M.R., Schaffer A. Biochemistry of metallothionein. Biochemistry 1988; 27: 8509–8515
- Garcia Chillon J.C., Martin Mateo M.C. Valoration of metallothionein in plasma kidney patients. Universidad de Valladolid Spain. 1996
- Biological Roll of Metallothionein, E.C. Foulkes. Elsevier Scientific Publishing Inc., New York 1982
- Garret S.H., Sens M.A., Todd J.H., Somji S., Sense D. A. Expression of MT in the human kidney. Toxicol Letters 1999; 105(3)207–214
- Rodilla V., Miles A.T., Jenner W., Hawksworth G.M. Exposed of cultural human proximal tubular cells to Cd, Hg, and Zinc: toxicity and met induction. Chem. Bio. Interact 1999; 115(i)71–73
- Martin Mateo M.C., del Canto E., Barrero M.J. Oxidative stress and enzyme activity in ambulatory renal patients. Renal Failure 1998; 20: 117–124
- Perez Fernandez R., deVega L., Martín Mateo M.C., Bustamante J. Study of antioxidants enzymes and lipid peroxidation in renal transplant. Renal Failure 2002; 24: 353–359
- Scheuhammer A.M., Cherian M.G. Quantification of metallothionein by silver saturation. Methods Enzymol. 1991; 205: 78–83
- Sciavon G.L., Biasioli S., De Fanti E., Targa L. Plasma glutation peroxidase activity as an index of renal function. Eur. J. Chem. Clin. Biochem. 1994; 32: 759–765