Abstract
Background. The incidence of extrapulmonary tuberculosis is higher in dialysis than general population. The aim of the study was to characterize clinical picture in dialysis patients, who developed extrapulmonary tuberculosis. Methods. We retrospectively investigated the hemodialysis patients with extrapulmonary tuberculosis. 2208 hemodialysis patients were reviewed for extrapulmonary tuberculosis from 10 1986 to 01 2001. Results. Seventeen patients (10 male, 7 female) were enrolled. The mean age was 57.4 ± 12.4 years. The sites for extrapulmonary tuberculosis were peritoneum (35.3%, 6/17), cervical lymph node (17.6%, 3/17), bone marrow (5.9%, 1/17), spine (5.9%, 1/17), knee (5.9%, 1/17), brain (5.9%, 1/17), pericardium (5.9%, 1/17), cutaneous tissue (5.9%, 1/17) and genitourinary system (5.9%, 1/17). Fourteen of 15 tissue-biopsy specimens from suspicious sites revealed granulomatous inflammation. There were low yield in mycobacteria culture (11.1%, 1/9) and PCR (33.3%, 2/6). Three patients died during the treatment of the disease. Conclusion. Extrapulmonary tuberculosis constitutes a major part of tuberculosis in dialysis patients. Tissue biopsy with invasive procedures, such as laparoscopy or laparotomy, may be necessary if clinical presentations are suspicious.
Introduction
Infectious disease is a common cause of morbidity and mortality in patients with end-stage renal disease on maintenance hemodialysis.Citation[[1]], Citation[[2]], Citation[[3]] The incidence of tuberculosis is increased in these patients due to impaired cellular immunity in renal failure patients.Citation[[4]], Citation[[5]], Citation[[6]] Extrapulmonary tuberculosis, which is very rare in general population, constitutes more than 50% of tuberculosis in patients under maintenance dialysis.Citation[[5]] Many other studies revealed the same phenomenon.Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]], Citation[[11]], Citation[[12]], Citation[[13]], Citation[[14]], Citation[[15]], Citation[[16]], Citation[[17]], Citation[[18]] The unusual presentation of the tuberculosis raises the question if there is special presentation of extrapulmonary tuberculosis in patients under maintenance hemodialysis. We reviewed 17 maintenance hemodialysis patients who were found to have sole extrapulmonary tuberculosis to understand the clinical characters of these patients. The characterization of these patients will surely improve our understanding of the disease and reminds us this uncommon complication in hemodialysis patients.
Patients and Methods
We retrospectively reviewed the patients with extrapulmonary tuberculosis from a database of a 2208 chronic hemodialysis patients pool in one single medical school affiliated hospital during October 1986 to January 2001. Seventeen patients with sole extrapulmonary tuberculosis were enrolled into the study. Ten of them are male and 7 are female. The underlying renal disease, duration of dialysis, clinical presentations, biochemistry data, microbiology study, treatment and outcomes of the patients were analyzed.
Chest X-ray, sputum smear, tissue biopsy (Ziehl-Neelsen's stain), mycobacteria-tuberculosis culture (Lowenstein-Jensen media) or polymerase chain reaction (PCR) for acid-fast bacilli had been performed for all suspected cases.
Extrapulmonary tuberculosis was diagnosed in patients without active pulmonary lesion and with any of the following criteria: (1) tissue biopsy demonstrating granulomatous inflammation and/or positive acid-fast bacilli stain; (2) positive mycobacteria-tuberculosis culture; (3) positive mycobacteria-tuberculosis PCR.
Isoniazid 100–300 mg daily, rifampicin 450–600 mg daily, ethambutol 400–800 mg daily or pyrazinamide 750–1500 mg daily were given in combination for more than 1 year in every patients. Ethambutol was spared in some patients to avoid possible retrobulbar neuropathy. Patients were given pyridoxine 50 mg daily together with isoniazid to prevent the neurological adverse effect. All data was presented as mean ± SD.
Results
Thirty-three patients were found to have tuberculosis during the study period. Sixteen of them developed solely pulmonary tuberculosis. Clinical characteristics for the other 17 patients presenting with extrapulmonary tuberculosis are summarized in . None of these patients received previous renal transplantation. All the patients did not received immunosuppressant therapy except one with systemic lupus erythematosus. The mean age for patients at diagnosis was 57.4 ± 12.4 years (range from 34 to 75). The duration of hemodialysis prior to the onset of extrapulmonary tuberculosis was 28.0 ± 32.6 months (range from 1 to 120). Underlying etiologies for end-stage renal disease were chronic primary glomerulonephritis (58.8%, 10/17), diabetes mellitus (35.3%, 6/17) and systemic lupus erythematous (5.9%, 1/17). The sites for the extrapulmonary tuberculosis were peritoneum (35.3%, 6/17), cervical lymph node (17.6%, 3/17), bone marrow (11.8%, 2/17), spine (5.9%, 1/17), knee (5.9%, 1/17), brain (5.9%, 1/17), pericardium (5.9%, 1/17), cutaneous tissue (5.9%, 1/17) and urinary tract (5.9%, 1/17). Nine patients developed extrapulmonary tuberculosis less than one year after diagnosis of end-stage renal disease. The presenting symptoms and signs were fever (35.3%, 6/17), abdominal fullness (35.3%, 6/17), conscious disturbance (11.8%, 2/17), cervical lymphadenopathy (11.8%, 2/17), abdominal pain (5.9%, 1/17), bone pain (11.8%, 2/17), knee pain (5.9%, 1/17), chest pain (5.9%, 1/17), and skin rash (5.9%, 1/17). Laboratory studies revealed hypercalcemia (64.7%, 11/17), hypo-albuminemia (47.1%, 8/17) and leukocytosis (35.3%, 6/17). The mean serum-calcium level was 10.7 ± 1.7 mg/dl (range from 8.3 to 13.4 mg%). The mean serum albumin was 2.8 ± 0.6 g/dl (range from 1.5 to 3.6). The mean peripheral-leukocyte count was 11,423 ± 8.977/mm3 (range 4.800–43,000).
Table 1. Clinical characteristics of 17 maintenance hemodialysis patients with extrapulmonary tuberculosis
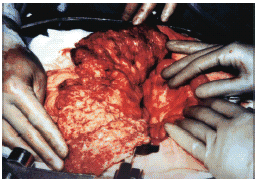
Fifteen patients received tissue biopsy in different organs other than lung for the diagnosis. The pathology revealed granulomatous inflammation, which suggested the tuberculosis, in 14 of them (93.3%). Only 5 of them were positive for acid-fast bacilli by Ziehl-Neelsen's stain. Urine from one of the patients grew up mycobacteria-tuberculosis in microbiology study. Ascites and cerebrospinal fluid from 2 of them had positive results from PCR for tuberculosis. Four of six patients presenting with abdominal fullness had received diagnostic laparotomy. Multiple adhesions between bowel loops were discovered for patient 5 after laparotomy (). Four patients had history of pulmonary tuberculosis with adequate therapy from 3–4 years before the onset of their extrapulmonary tuberculosis. However, the sputum smears and cultures were negative at the time of extrapulmonary tuberculosis.
Figure 1. Exploratory laparotomy revealing multiple adhesions between bowel loops. The miliary nodules were throughout the abdominal cavity including bowel wall, omentum, and mesentery.
All patients received anti-tuberculosis therapy. Twelve of them were treated with a three-drug combination and five were with a combination of four drugs. The majority of the patients tolerated the therapy very well. Isoniazid was discontinued after six months for the development of progressive legs numbness in one patient. Another patients had a progressive blur vision, which limited the continuous usage of ethambutol after eight months of therapy. One patient died on superimposed severe streptococcus Group D meningitis. Two patients with peritoneal tuberculosis died as a consequence of upper-gastrointestinal bleeding and severe intra-abdominal adhesion.
Discussion
Infectious disease continues to be a serious complication for maintenance dialysis patients.Citation[[18]], Citation[[19]], Citation[[20]] Host resistance to Mycobacteria tuberculosis is mediated by cellular immunity, which is impaired in renal failure patients.Citation[[5]], Citation[[6]] The incidence of tuberculosis was greatly increased in renal failure patients with an incidence from 10 to 20%.Citation[[7]], Citation[[8]], Citation[[9]], Citation[[13]], Citation[[14]], Citation[[15]], Citation[[21]], Citation[[22]], Citation[[23]], Citation[[24]], Citation[[25]] The incidence of tuberculosis is also affected by geographical, racial and social difference in different parts of world. The annual incidence of tuberculosis in dialysis patients was 6.9 times higher than general population in Taiwan in 2001.Citation[[21]] Furthermore, the incidences of extrapulmonary tuberculosis in these dialysis patients with tuberculosis is much higher than general population and were more than 50% in several studies.Citation[[5]], Citation[[15]], Citation[[21]] There were 16 patients in our dialysis population developed pulmonary tuberculosis during the study period. Extrapulmonary tuberculosis constitutes 51.5% (17/33) in our patients with tuberculosis. Peritoneum was the preferred site (35.3%) for our patients with extrapulmonary tuberculosis. The result is different from other studies, which always indicated lymphadenopathy was the major presentation.Citation[[13]], Citation[[15]], Citation[[17]] We believed that the difference might come from the difference of patient population. Our hospital is a tertiary hospital, which contains many critical patients and patients having previous abdominal operation. Five of 6 patients, who developed peritoneal tuberculosis, had a previous abdominal surgery. The previous abdominal surgical intervention might precipitate the occurrence of tuberculosis.
Consistent with a previous report,Citation[[14]] most of patients (52.9%, 9/17) developed extrapulmonary tuberculosis less than one year after diagnosis of end-stage renal disease. It was likely that stable and adequate dialysis would enhance the patient immunity and decrease the possibility to get tuberculosis in dialysis patients. The presenting symptoms were nonspecific and might indicate the sites of the extrapulmonary tuberculosis.Citation[[8]], Citation[[9]], Citation[[14]] The classic low-grade fever appeared in only 6 of our patients (35.3%). Hypercalcemia and hypoalbuminemia were the most common laboratory findings in these patients. The combined appearance of these features might be a predictor of development of the infection. Tuberculosis peritonitis was noted in six patients. All of them presented with sensation of abdominal fullness. Ascites was not a constant feature and the diagnosis was confirmed by laparotomy or laparoscopy in these six patients.
Tissue biopsy with typical granulomatous pathological change might be the most reliable diagnostic tool for the disease. The positive rate of PCR was only 33.3% (2 in 6 patients). The yield of culture was not satisfactory either. Ascites culture failed to grow any acid-fast bacilli in 5 of 6 patients with ascites, a finding consistent with previous reports.Citation[[8]], Citation[[10]] We suggest invasive procedure with tissue biopsy may be necessary when clinical presentations are highly suspicious.
Diabetic patients were prone to infection in many studies.Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]] In our dialysis unit, 30.5% of the patients were diabetic. Six (35.3%) of our extrapulmonary tuberculosis patients were diabetic. There is no diabetic predominance in this specific patient group. Previous tuberculosis history was only present in 4 of these patients. Mortality was up to 17.6% (3/17) due to complications during anti-tuberculosis therapy. We believed early diagnosis and treatment would improve the patient outcome.
We conclude that extrapulmonary tuberculosis constituted a major part of tuberculosis in maintenance hemodialysis patients. There were no specific signs and symptoms for the disease. Tissue biopsy with invasive procedures, such as laparoscopy and or laparotomy, may be necessary if clinical presentations are particularly suspicious.
References
- Mailloux L.U., Bellucci A.G., Wikes B.M., Napolitano B., Mossey R.T., Lesser M., Bluestone P.A. Mortality in dialysis patients: analysis of the causes of death. Am. J. Kidney Dis. 1991; 18: 326–335
- Fernandez J.M., Carbonell M.E., Mazzuchi N., Petruccelli D. Simultaneous analysis of morbidity and mortality factors in chronic hemodialysis patients. Kidney Int. 1992; 41: 1029–1034
- Churchill D.N., Taylor W., Cook R.J., Laplante P., Barre P., Cartier P., Fay W.P., Goldstein M.B., Jindal K., Mandin H., Mckenzie J.K., Muirhead N., Parfrey P.S., Posen G.A., Slaughter D., Ulna R.A., Werb R. Canadian hemodialysis morbidity study. Am. J. Kidney Dis. 1992; 19: 214–234
- Wilson W.E.C., Kirkpatrick C.H., Talmage D.W. Suppression of immunologic responsiveness in uremia. Ann. Intern. Med. 1965; 62: 1–8
- Tolkoff-Rubin N.E., Rubin R.H. Uremia and host defense. N. Engl. J. Med. 1990; 322: 770–772
- Vanholder R., Ringoir S., Dhondt A., Hakin R. Phagocytosis in uremic and hemodialysis patients: a prospective and cross section study. Kidney Int. 1990; 39: 320–327
- Pradhan R.P., Katz L.A., Nidus B.D., Matalonn R., Eisinger R.P. Tuberculosis in dialysis. JAMA 1974; 229: 798–802
- Lundin A.P., Adler A.J., Berlyne G.M., Friedman Eli A. Tuberculosis in patients undergoing maintenance hemodialysis. Am. J. Med. 1979; 67: 597–602
- Sasaki S., Akiba T., Suenaga M., Tomura S., Yoshiyama N., Nakagawa S., Shoji T., Sasaoka T., Takeuchi J. Ten-year survey of dialysis associated tuberculosis. Nephron 1979; 24: 141–146
- Papadmitriou M., Memmos D., Metaxas P. Tuberculosis in patients on regular hemodialysis. Nephron 1979; 24: 53–57
- Andrew O.T., Schoenfeld P.Y., Hopewell P.C. Tuberculosis in patients with end-stage renal disease. Am. J. Med. 1980; 68: 59–65
- Beclcon M.C., Smith E.K.M., Kahana L.M., Shimizu A.G. Tuberculosis in dialysis patients. Clin. Nephrol. 1982; 17: 14–18
- Rieder H.L., Snider D.E., Jr., Cauthen G.M. Extrapulmonary tuberculosis in the United State. Am. Rev. Respir. Dis. 1990; 141: 347–351
- Hussein M.M., Bakir N., Roujouleh H. Tuberculosis in patients undergoing maintenance dialysis. Nephrol. Dial. Transplant. 1990; 5: 584–587
- Mitwalli A. Tuberculosis in patients on maintenance dialysis. Am. J. Kidney Dis. 1991; 5: 579–582
- Al-Kassimi F.A. Review of tuberculosis in Saudi Arabia. Saudi Med. J. 1994; 15: 192–195
- Al-Homrany M. Successful therapy of tuberculosis in hemodialysis patients. Am. J. Nephrol. 1997; 17: 32–35
- Shohaib S.A., Scrimgeour E.M., Shaerya F. Tuberculosis in active dialysis patients in Jeddah. Am. J. Nephrol. 1999; 19: 34–37
- Montgomerie J.Z., Kalmanson G.M., Guze L.B. Renal failure and infection. Medicine 1968; 47: 1–32
- Keane W.F., Shapiro F.L., Raij L. Incidence and type of infection occurring in 445 chronic hemodialysis patients. ASAIO Trans. 1977; 23: 41–47
- Chou K.J., Fang H.C., Bai K.J., Hwang S.J., Yang W.C., Chung H.M. Tuberculosis in maintenance dialysis patients. Nephron 2001; 88: 138–143
- Smith F.W., Catto G.R.D., MacLeod M. Treatment of pulmonary tuberculosis in a patient on maintenance hemodialysis. Postgrad. Med. J. 1974; 50: 478–481
- Keane W.F., Raij L. Infection complications in maintenance dialysis patients. Replacement of Renal Function by Dialysis, Drukker, Parsons, Maher. Nijhoff, The Hague 1978; 621
- Freeman R.M., Newhouse C.E., Lawton R.L. Absence of tuberculosis in dialysis patients (letter). JAMA 1975; 233: 1356
- Ogg C.S., Toseland P.A., Cameron J.S. Pulmonary tuberculosis in patient on intermittent hemodialysis. Br. Med. J. 1968; 2: 283–284
