Abstract
The aim of this study was to compare the prevalence of anticardiolipin antibodies with other types of antiphospholipid antibodies (aPL) (antiphosphatidylserine—aPS, antiphosphatidylinositol—aPI, antiphosphatidylethanolamine—aPE) in patients with lupus nephritis and to find if the examination of a panel of various aPL is valuable for further diagnosis of patients. Additionally we determined the levels of autoantibodies against β2-glycoprotein I (β2GPI) and oxidized low-density lipoprotein (anti-oxLDL) and also investigated the relationship between antibodies against β2GPI and oxLDL, which were assessed by ELISA methods. Twenty-two patients with lupus nephritis were studied. The control group consisted of 62 healthy blood donors. A statistically significant higher occurrence of all aPLs in the patients with lupus nephritis in comparison to the control group was found. The prevalence of polyspecific antibodies, which reacted with at least two various phospholipids, was 82% in the group of SLE patients. Significantly higher levels of IgG anti-β2GPI in the sera of SLE patients (p = 0.0003) was detected. The levels of anti-oxLDL in the sera of the patients group did not differ significantly from the control one. Some positive samples for anti-β2GPI and negative for aCL or anti-oxLDL and vice versa were found. It can be concluded that the production of aPL including anti-β2GPI and anti-oxLDL in the lupus nephritis patients is higher in comparison with healthy blood donors. We assume that the estimation of various types of aPL may be important in the selection of the group patients with renal diseases. The synthesis of aPL can reflect the spreading of the autoimmune response for several antigens modified on the vessel wall.
Introduction
Antiphospholipid antibodies (aPL) are a family of autoantibodies directed against different target antigens, predominantly anionic phospholipid or phospholipid-containing structures. Cardiolipin is the antigen most commonly used in antiphospholipid antibody assay. Besides cardiolipin other phospholipids such as phosphatidylserine, phosphatidylinositol or phosphatidic acid or zwitterionic phosphatidylethanolamine have been used in ELISA.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]] Negatively charged phospholipids were tested separately and as a mixture to determine which would best distinguish antiphospholipid syndrome (APS) sera from anticardiolipin-positive nonAPS samples.Citation[[5]] Gharavi et al.Citation[[1]] found that there was no significant difference between antibody binding to cardiolipin (aCL) and binding to other anionic phospholipid—phosphatidylserine and phosphatidylinositol, suggesting that the specificity of these antibodies is for negatively charged phospholipids in general, rather than for cardiolipin in particular. Antibodies reacting with dipolar ionic phosphatidylcholine and phosphatidylethanolamine were also described in specific conditions.Citation[[3]], Citation[[6]]
The antiphospholipid antibodies may react not only with several different phospholipid structures. Most of the aPL requires the presence of phospholipid-binding proteins such as β2-glycoprotein I (β2GPI) or prothrombin.Citation[[7]], Citation[[8]] It is now widely accepted that β2GPI is a target antigen for these autoantibodies.Citation[[9]], Citation[[10]]
β2GPI is a natural anticoagulant in vitro. It inhibits the contact activation of the intrinsic coagulation pathways, prothrombinase activity, and ADP-induced platelet aggregation.Citation[[11]], Citation[[12]], Citation[[13]] Much of the in vitro anticoagulant activity of β2-glycoprotein I results from the binding of this protein to negatively charged phospholipid surfaces, e.g., activated platelets.Citation[[14]] The binding of β2GPI competitively inhibits the binding of some coagulation factors and the prothrombinase complex, thereby preventing the activation of the coagulation cascade.Citation[[15]] However, Harper et al.Citation[[16]] suggested a lack of anticoagulant effect of β2GPI under near physiological conditions with a relatively weak binding to plasma membranes. It is possible that β2GPI may be principally effective in abnormal conditions such as inflammation or oxidative stress. Recently Price et al.Citation[[17]] have demonstrated that β2-glycoprotein I bind apoptotic cells because they lost the membrane phospholipid asymmetry allowing the exposure of phosphatidylserine. Therefore antibodies against β2-glycoprotein I might well bind in vivo to the surface of apoptotic cells.
Antibodies reacting with cardiolipin and anionic phospholipids directly can be infection-associated and cause positive reactions in serological tests for syphilis. Autoimmune types of aCL are directed against a complex consisting of phospholipid and β2GPI rather than phospholipids alone.Citation[[18]]
Some aPL can be directed against epitopes common with oxidized low-density lipoprotein (oxLDL). Antibodies to oxidized LDL (anti-oxLDL) comprise antibodies recognizing oxidized lipids, probably responsible for the cross-reactivity with cardiolipin and antibodies that bind to oxidized apolipoprotein B.Citation[[19]] Hasunuma et al.Citation[[20]] reported that β2-glycoprotein I directly bound to oxidized plasma lipoproteins and that the complex of oxLDL and β2-glycoprotein was subsequently recognized by aCL. Some of the antibodies binding to oxidized LDL may actually be antibodies to β2-glycoprotein I that recognize their antigen in association with LDL.Citation[[21]] These antibodies may represent a separated group, although some antibodies seem to be cross-reactive with antibodies binding to cardiolipin.Citation[[19]]
The aim of this study was to compare the prevalence of anticardiolipin antibodies, which are commonly examined with other types of aPL in patients with lupus nephritis, and to find if the estimation of a panel of various aPL is valuable for diagnosis of patients with renal diseases. Furthermore, we estimated the levels of autoantibodies against β2GPI and oxLDL and also evaluated the relationship between antibodies against β2-glycoprotein I and oxLDL.
Material and Methods
Patients
We studied 22 patients with lupus nephritis. The mean age of the patients was 36 ± 12 years. None of the patients had signs of renal failure or advanced renal insufficiency. The diagnosis was based on clinical and histopathologic criteria. Control group consisted of the 62 healthy blood donors. Sera were freshly frozen and stored at −20°C. All samples were measured together. All patients have given their informed consent prior to entering the study.
Determination of IgG and IgM Antibodies Against Various Phospholipids
Serum samples were evaluated for IgG and IgM antibodies against cardiolipin (aCL), phosphatidylserine (aPS), phosphatidylinositol (aPI), and phosphatidylethanolamine (aPE) using enzyme-linked immunoassay according to the modification of Harris.Citation[[22]] Briefly, individual 96-well microtiter plates (Polysorp, Nunc International, Roskilde, Denmark) were coated with 1 µg/well of each phospholipid (l-α-phosphatidyl-l-serine, l-α-phosphatidylinositol, l-α-phosphatidylethanolamine (Sigma Chemicals, St. Louis, MO) dissolved in ethanol or in a mixture of methanol–chloroform. The liquid was allowed to evaporate overnight at 4°C. Blocking with 10% adult bovine serum in phosphate buffered saline pH was performed for 30 min at 37°C. After washings, sera diluted 1:50 were added to the wells and incubated for 2 h at 37°C. The plates were washed three times and then were incubated with horseradish peroxidase-conjugated goat antihuman IgG and IgM (Sevapharma, Prague, CR) and then the plates were washed. Color was developed by adding a substrate (o-phenylendiamine solution with H2O2) for 15–20 min at a room temperature. The enzymatic reaction was stopped by adding 2 mol/L H2SO4. The absorbance of each well was read at 495 nm by a microplate reader.
Antiphospholipid assays other than aCL have not been yet standardized and there is no standard material available for calibration. Known high positive and negative sera and our own internal standard were run on each plate. Positive reaction was defined as an absorbance greater than mean + 5 SD of 15 control subjects.
Determination of IgG Antibodies Against β2-Glycoprotein I and Cardiolipin
Sera were assayed for IgG anti-β2GPI by a commercial ELISA kit (ORGENTEC, Mainz, Germany) using the γ-irradiated polystyrene microtiter plates, which were coated with highly purified human β2GPI. Diluted patients (1:100) samples were pipetted into the wells and incubated for 30 min at a room temperature. After washings, an anti-human-IgG horseradish peroxidase conjugate solution was added into each well and incubated for 15 min at a room temperature. The enzyme conjugate, not specifically bound, was washed and a substrate (3,3′, 5,5′-tetramethylbenzidin) was added. After 15 min of incubation at a room temperature the color reaction was stopped by adding hydrochloric acid solution. Samples were considered positive if the levels of antibodies exceeded 6.3 U/mL (mean + 5SD of 25 normal controls).
Anticardiolipin antibodies were determined by a standardized ELISA, utilizing cardiolipin as an antigen (IMMCO Diagnostics, Inc., Buffalo, USA). The results are expressed in units (U/mL) assigned as GPL for IgG class. The results have been interpreted as positive if the levels exceeded 17 GPL (mean + 5SD of 25 normal controls).
Determination of IgG Antibodies Against Oxidized Low-Density Lipoprotein
To determinate anti-oxLDL we used anti-oxLDL-ELISA kit (Biomedica, Vienna, Austria). The microtiter strips were coated by Cu2+ oxidized LDL as an antigen. Prediluted serum samples (1:50) were incubated for 90 min at 37°C. After washings, the anti-human IgG antibody conjugated with a peroxidase was added to each well and incubated for 30 min at a room temperature. The wells were washed, the substrate was added and the microplate was incubated for 15 min at a room temperature. Adding sulphuric acid stopped the enzymatic reaction.
The levels of IgG anti-oxLDL higher than 760 mU/mL (mean + 5SD of 62 normal controls) were considered to be positive.
Statistical Methods
Means and standard deviations were calculated by conventional methods. To compare the results obtained for patients' group and control's group the test t-test of alternate separation and unpaired t-test was employed.
Results
The basic biochemical parameters of patients' renal functions are summarized in . No signs of renal failure or advanced renal insufficiency were recorded (glomerular filtration was above 1.0 mL/s).
Table 1. Basic biochemical parameters of renal functions in patients with lupus nephritis
shows the occurrence of various IgG and IgM aPL in the group of patients. We found a statistically significant higher incidence of all tested aPL in the patients with lupus nephritis in comparison with the control group. summarizes the presence of aPL with various phospholipid specificity.
Table 2. Occurrence of various types of aPL in patients with lupus nephritis
Table 3. Occurrence of aPL in the patients with lupus nephritis
The presence of polyspecific aPL which reacted with at least two various phospholipids and aPL directed against one or more phospholipids with the exception of cardiolipin (non-aCL) was 82% in the group of lupus nephritis patients and 23% for non-aCL.
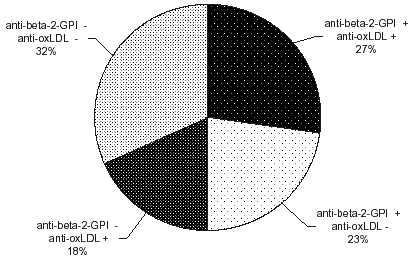
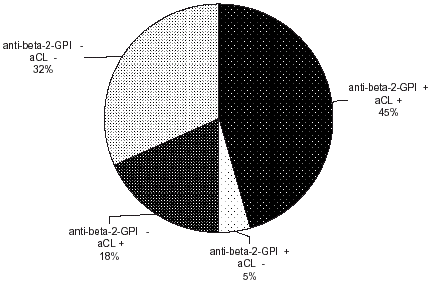
We found significantly higher levels of IgG anti-β2GPI in patients (p<0.001). The levels of anti-oxLDL did not differ significantly in the patients from control group (). Some positive samples for anti-β2GPI IgG and negative for aCL or anti-oxLDL and vice versa exist ( and ).
Table 4. Serum levels of anti-β2GPI IgG and anti-oxLDL IgG in patients with lupus nephritis
Discussion
Antiphospholipid antibodies are associated with a well-defined clinical condition known as the antiphospholipid syndrome (APS), which are characterized by arterial and venous thrombotic events, recurrent abortions, and thrombocytopenia.Citation[[23]]
The most commonly described aPL is aCL.Citation[[24]] Cardiolipin is a component of the mitochondria and is not found on mammalian cell membranes. Because phospholipids such as phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol are more widely distributed in the cell membranes than cardiolipin, the examination of presence of various antiphospholipid antibodies may be interesting in understanding of their role in some disorders.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[25]], Citation[[26]]
Phosphatidylserine is a negatively charged phospholipid with similar phosphatidyl moieties such as cardiolipin. It appears to be a more physiological autoantigen than cardiolipin for the detection of aPL antibodies. In contradistinction to cardiolipin, phosphatidylserine is generally located at the inner side of plasma membranes, but it is also found on the outer surface of cellular membranes of platelets and endothelial cells.Citation[[27]] Phosphatidylserine plays role in the coagulation cascade and has a function in clot formation.Citation[[28]] The occurrence of aPL directed against further anionic phospholipid phosphatidylinositol could be associated with thromboembolic events or recurrent fetal loss.Citation[[2]]
Autoantibodies directed to zwitterionic phospholipids have not been studied intensively in the course of various diseases. Phosphatidylethanolamine is a major component of both the outer and inner leaflet of cell plasma membranes. aPE were found in autoimmune patients with thrombosis and have even been reported as the sole aPL in sera of some patients.Citation[[3]]
In patients with lupus nephritis we looked for aPL reactive not only with cardiolipin but also with other anionic phospholipids such as phosphatidylserine, phosphatidylinositol, and with the zwitterionic phosphatidylethanolamine.
In patients with lupus nephritis we found high titers of all tested autoantibodies and also the occurrence of polyspecific aPL was 82%. The frequency of non-aCL was 23%. It is probable that individual patients may produce a mixture of antibodies of different isotypes reacting with both negatively charged and zwitterionic phospholipids. Our results confirm a considerable dysregulation of the immune system in patients with SLE.
Antiphospholipid antibodies are immunoglobulins, which are closely related but probably have distinct bindings affinities. β2-glycoprotein I was defined as a co-factor for anticardiolipin antibodies. It is possible that aPL recognize a complex formed by cardiolipin and β2-glycoprotein I or a cryptic epitope exposed on β2GPI molecule after interaction with phospholipids or exposure to cryptic epitope on cardiolipin upon binding to β2GPI which leads to generation of pathogenic aCL.Citation[[9]] Some investigations have shown that epitopes on the fifth domain of β2GPI are the real target of aCL—even in absence of negatively charged phospholipids.Citation[[29]]
Some aPL are in fact directed to epitopes of oxidized phospholipids, or neoepitopes generated by adduct formation between breakdown products of oxidized phospholipids and associated proteins (including β2GPI).Citation[[21]] A small amount of plasma β2GPI is bound to the LDL molecule in circulation and therefore antibodies to β2GPI may show binding to oxidized LDL.Citation[[30]] It is known that some antibodies to oxidized LDL bind to oxidized lipids in the LDL molecule and is likely responsible for cross-reactivity with phospholipids such as cardiolipin.Citation[[31]], Citation[[32]]
The finding of the statistically significant high levels of anti-β2GPI in the lupus nephritis patients is concordant with the literature. Anti-β2GPI are associated with a risk for both arterial and venous thrombosis in SLE. IgG anti-β2GPI were found in 36% of SLE patients and the high titers were strongly associated with a history of arterial thrombosis.Citation[[37]], Citation[[38]] In our lupus nephritis patients we also found significantly higher titers of anti-β2GPI IgG. The elevated levels of anti-β2GPI in SLE can be explained by the fact that besides their effect on blood coagulation, antibodies to β2GPI can enhance the uptake of oxLDL bound with β2-glycoprotein I into macrophages.Citation[[20]] It is possible that the production of antiphospholipid antibodies is initiated as a consequence of the underlying systemic arterial inflammatory disease and reflect the degree of the inflammatory process.
The levels of anti-oxLDL in the sera of patients with lupus nephritis were higher than in controls but not statistically significant. Vascular inflammation together with immunological abnormalities is a basic feature of SLE. Furthermore SLE is associated with an increased risk of clinical coronary artery disease, which is accelerated in its onset.Citation[[33]] The mechanism of this accelerated atherosclerosis in SLE is unclear. Iuliano et al.Citation[[34]] found that patients with APS secondary to SLE have been shown to possess markers of lipid peroxidation. George et al.Citation[[35]] have found that mice actively immunized with human aPL developed mouse aPL that enhanced fatty streak formation. Shoenfeld et al.Citation[[36]] suppose that APS and atherosclerosis may share a common etiologic background, which may have direct implications on the management of both conditions.
We found some anti-β2GPI IgG without aCL or anti-oxLDL and vice versa. Therefore it is possible that two partially overlapping populations of antibodies exist—antibodies that react only with β2GPI or antibodies that react only with oxidized LDL and cross-reacting antibodies that bind both to β2GPI and to oxidize LDL. These antibodies with various specificities, which might have different biological effects, may occur in the same patient.
We can conclude that the production of aPL including anti-β2GPI and antibodies against oxLDL in the lupus nephritis patients is higher in comparison with healthy blood donors. We assume that the estimation of various types of aPL may be important in selecting group of patients with lupus nephritis. The intensity of synthesis of aPL can reflect the spreading of the autoimmune response against several antigens modified in the vessel wall.
Antiphospholipid Antibodies in Patients with Lupus Nephritis
E-mail Addresses
| Lenka Fialová: | = | |
| Tomáš Zima: | = | |
| Vladimir Tesař: | = | |
| Ludmila Mikulková: | = | |
| Ivan M. Malbohan: | = | |
| Miroslav.Merta: | = | |
| Věra Čertíková: | = |
Acknowledgment
This work was supported by research project No. 206017-01.
References
- Gharavi A.E., Harris E.N., Asherson R.A., Hughes G.R.V. Anticardiolipin antibodies: isotype distribution and phospholipid specificity. Ann. Rheum. Dis. 1987; 46: 1–6
- Falcon C.R., Hoffer A.M., Carreras L.O. Antiphosphatidylinositol antibodies as markers of the antiphospholipid syndrome. Thromb. Haemost. 1990; 63: 321–322
- Berard M., Chantome R., Marcelli A., Boffa M.C. Anti-phosphatidylethanolamine antibodies as the only antiphospholipid antibodies. I. Association with thrombosis and vascular cutaneous diseases. J. Rheumatol. 1996; 23: 1369–1374
- Branch D.W., Silver R., Pierangeli S., van Leeuwen I., Harris E.N. Antiphospholipid antibodies other than lupus anticoagulant and anticardiolipin antibodies in women with recurrent pregnancy loss, fertile controls, and antiphospholipid syndrome. Obstet. Gynecol. 1997; 89: 549–555
- Harris E.N., Pierangeli S.S., Gharavi A.E. Diagnosis of the antiphospholipid syndrome: a proposal for use of laboratory tests. Lupus 1998; 7(suppl 2)144–148
- Cabral A.R., Cabiedes J., Alacron-Segovia D. Hemolytic anemia related to an IgM autoantibody to phosphatidylcholine that binds in vitro to stored and to bromelain-treated human erythrocytes. J. Autoimmun. 1990; 3: 773–787
- Roubey R.A.S., Eisenberg R., Harper M.F., Winfield J.B. Anticardiolipin autoantibodies recognize β2-glycoprotein I in the absence of phospholipid: importance of antigen density and bivalent binding. J. Immunol. 1995; 154: 954–960
- Bevers E.M., Galli M., Barbui T., Comfurius P., Zwaal R.F. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb. Hemost. 1991; 66: 629–632
- Shoenfeld Y., Gharavi A., Koike T. β2GPI in the antiphospholipid (Hughes′) syndrome—from a cofactor to an autoantigen—from induction to prevention of antiphospholipid syndrome. Lupus 1998; 7: 503–506
- Sheng Y., Kandiah D.A., Krilis S.A. β2-Glycoprotein I: target antigen for antiphospholipid antibodies. Immunological and molecular aspects. Lupus 1998; 7(suppl 2)S5–S9
- Schousboe I. Beta-2-glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway. Blood 1985; 66: 1086–1091
- Nimpf J., Bevers E.M., Bomans P.H., Till V., Wurm H., Kostner G.M., Zwaal R.F. Prothrombinase activity of human platelets is inhibited by β2-glycoprotein I. Biochim. Biophys. Acta 1986; 884(1)142–149
- Nimpf J., Wurm H., Kostner G.M. β2-Glycoprotein I (apo-H) inhibits the release reaction of human platelets during ADP-induced aggregation. Atherosclerosis 1987; 63: 109–114
- Shi W., Chong B.H., Chesterman C.N. β2-Glycoprotein I is a requirement for anticardiolipin antibodies binding to activated platelets: differences with lupus anticoagulants. Blood 1993; 81: 1255–1262
- Chamley L.W. Antiphospholipid antibodies or not? The role of β2-glycoprotein I in autoantibody-mediated pregnancy loss (review). J. Reprod. Immunol. 1997; 36: 123–142
- Harper M.F., Lentz B.R., Roubey R.A.S. β2-Glycoprotein I (β2GPI) binding to phospholipid (PL) membranes. Lupus 1996; 5: 522
- Price B.E., Rauch J., Shia M.A., Walsh M.T., Lieberthal W., Gilligan H.M., o'Laughalin T., Koh J.S., Levine J.S. Anti-phospholipid antibodies bind apoptotic but not to viable, thymocytes in a β2-glycoprotein I—dependent manner. J. Immunol. 1996; 157: 2201–2208
- Matsuura E., Igarashi Y., Fujimoto M., Ichikawa K., Koike T. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet 1990; 336: 177–178
- Vaarala O., Puurunen M., Lukka M., Alfthan G., Leirisalo-Repo M., Aho K., Palosuo T. Affinity-purified cardiolipin-binding antibodies show heterogeneity in their binding to oxidized low-density lipoprotein. Clin. Exp. Immunol. 1996; 104(2)269–274
- Hasunuma Y., Matsuura E., Makita Z., Katahira T., Nishi S., Koike T. Involvement of β2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin. Exp. Immunol. 1997; 107(3)569–673
- Hörkkö S., Miller E., Branch D.W., Palinski W., Witztum J.L. The epitopes for some antiphospholipid antibodies are adducts of oxidized phospholipid and β2-glycoprotein I (and other proteins). Proc. Natl. Acad. Sci. USA 1997; 94: 10356–10361
- Harris E.N. Antiphospholipid antibodies. Br. J. Hematol. 1990; 74: 1–9
- Hughes G.R. Hughes′ syndrome: the antiphospholipid syndrome. A historical view. Lupus 1998; 7(suppl 2)S1–S4
- Piette J.C. Towards improved criteria for the antiphospholipid syndrome. Lupus 1998; 7(suppl 2)S149–S157
- Fialová L., Mikulíková L., Malbohan I.M., Benešová O., Zwinger A. Prevalence of various antiphospholipid antibodies in pregnant women. Physiol. Res. 2000; 49: 299–305
- Zima T., Fialová L., Mikulíková L., Malbohan I.M., Popov P., Nešpor K. Antibodies against phospholipids and oxidized LDL in alcoholic patients. Physiol. Res. 1998; 47: 351–355
- McNeil H.P., Chesterman C.N., Krilis S.A. Immunology and clinical importance of anticardiolipin antibodies. Adv. Immunol. 1991; 49: 193–280
- Bevers E., Comfrius P., van Rijn J., Hemker C., Zwaal R. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 1982; 122: 429–436
- Hunt J., Krilis S. The fifth domain of β2-glycoprotein I contains a phospholipid binding site (Cys 281–Cys 288) and a region recognized by anticardiolipin antibodies. J. Immunol. 1994; 52: 653–659
- Matsuura E., Katahira T., Igarashi Y., Koike T. β2-Glycoprotein I bound to oxidatively modified lipoproteins could be targeted by anticardiolipin antibodies. Lupus 1994; 3: 314–317
- Hörkkö S., Miller E., Dudl E., Reaven R.V., Curtiss L.K., Zvaifler N., Terkeltaub R., Pierangeli S.S., Branch D.W., Palinski W., Witztum J.L. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipid. J. Clin. Invest. 1996; 98(3)815–825
- Vaarala O. Autoantibodies to modified LDLs and other phospholipid–protein complexes as markers of cardiovascular diseases. J. Inter. Med. 2000; 24: 381–384
- Urowitz M.B., Gladman D.D. Accelerated atheroma in lupus—background (review). Lupus 2000; 9: 161–165
- Iuliano L., Pratico D., Ferro D., Pittoni V., Valesini G., Lawson J., FitzGerald G.A., Violi F. Enhanced lipid peroxidation in patients positive for antiphosholipid antibodies. Blood 1997; 90: 3931–3935
- George J., Afek A., Gilburd B., Levy Y., Blank M., Kopolovic J., Harats D., Shoenfeld Y. Atherosclerosis in LDL receptor knockout mice is accelerated by immunization with anticardiolipin antibodies. Lupus 1997; 6(9)723–729
- Shoenfeld Y., Harats D., George J. Atheroslerosis and the antiphospholipid syndrome. A link unravelled. Lupus 1998; 7(suppl 2)S140–S143
- Viard J.P., Amoura Z., Bach J.F. Association of anti-β2-glycoprotein I antibodies with lupus type circulating anticoagulant and thrombosis in systemic lupus erythematosus. Am. J. Med. 1992; 93: 181–186
- Romero F.I., Amengual O., Atsumi T., Khamashta M.A., Tinahones F.J., Hughes G.R. Arterial disease in lupus and secondary antiphospholipid syndrome: association with anti-β2-glycoprotein I antibodies but not with antibodies against oxidized low-density lipoprotein. Br. J. Rheumatol. 1998; 37: 883–888