Abstract
Aim. Hyperhomocystinemia is now recognized as an independent risk factor for atherosclerotic cardiovascular disease in patients with normal renal function. Hyperhomocystinemia is common in patients with chronic renal failure. This aim of this study was designed to look for associations between hyperhomocystinemia and clinically symptomatic atherosclerotic vascular disease (ASVD) in Taiwanese chronic hemodialysis patients. Methods. Two hundred patients undergoing hemodialysis were enrolled in the study. They had predialysis blood work performed for total homocysteine, serum folate, and vitamin B12 levels. A history of clinically significant ASVD was elicited using information from the patients' questionnaires and verified by careful inpatient and outpatient chart review. Results. Mean homocysteine concentration was 29.0 µmol/L overall. A total of 196 patients had hyperhomocystinemia and were enrolled in this study. ASVD was present in 24.5% of patients. The mean homocysteine concentration was 31.9 µmol/L and 28.7 µmol/L in patients with (n = 48) and without (n = 148) ASVD, respectively (P = 0.04). There was association hyperhomocystinemia between chronic hemodialysis patients with ASVD and without ASVD. There were significant differences including age, homocysteine level, vitamin B12 level and glucose intolerance in the two groups (P<0.05). Plasma homocysteine concentrations showed significant positive correlations with serum folate or vitamin B12 in majority patients. No patient had serum folate or vitamin B12 deficiency. The adjusted odds ratio for atherosclerotic disease was 2.8 (95%CI, 1.109–7.467) for those subjects with a homocysteine level in the highest quartile compared with the lowest 3 quartiles. Conclusions. The prevalence of hyperhomocystinemia is 98% among hemodialysis patients. There is a significant association between hyperhomocystinemia and ASVD in Taiwanese chronic hemodialysis patients. There are also associations between homocysteine levels and serum folate/vitamin B12 levels in the majority of the patients. Clinical trials are needed to determine the optimal therapy of folic acid dose for hyperhomocystinemia in Taiwanese chronic hemodialysis patients in the future.
Introduction
Hyperhomocystinemia is a common finding in patients with end-stage renal disease (ESRD)Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]], Citation[[5]], Citation[[6]], Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]] and is a risk factor for arthrosclerotic complications in these patients.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]], Citation[[5]], Citation[[6]], Citation[[7]], Citation[[8]] The mechanisms of hyperhomocystinemia in patients with ESRD are unclear and may include relative or absolute deficiencies of folic acid, vitamin B12, or vitamin B6.Citation[[5]], Citation[[6]], Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]] Reduced renal metabolism of homocysteine has also been proposed.Citation[[11]] However, previous reportsCitation[[1]], Citation[[2]], Citation[[3]], Citation[[4]], Citation[[5]], Citation[[6]], Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]], Citation[[11]] in regards to hyperhomocystinemia in hemodialysis are primarily from western countries, and only a few are from Taiwan. In this study, we investigated the association between hyperhomocystinemia and clinically symptomatic ASVD in Taiwanese chronic hemodialysis patients.
Methods
A total of 200 patients received hemodialysis in our hospital from April 1999 to December 2001 in this study. This was a cross-sectional analysis of patients with ESRD receiving hemodialysis (less than 18 months duration) in a centralized dialysis unit. The underlying causes of ESRD in this population were as follows. Approximately 50% of the patients had diabetic nephrosis, 43% had chronic glomerulonephritis, and the remaining had other diseases. Exclusion criteria were refusal or inability to give informed consent and requirement for short-term (i.e., <6 months) dialysis. All study subjects provided written informed consent.
Ninety-nine percent (198/200) of patients were receiving vitamin B complex (2 mg vitamin B1, 2 mg vitamin B2, 1 mg vitamin B6, 1 mg vitamin B12, 2 mg Ca pantothenate, and 10 mg nicotinamide). Fifteen percent (30/200) of patients were receiving supplemental 5 mg of oral folic acid per week during the initial stages of this study. Midweek predialysis blood samples were collected from all participants for total homocysteine levels. All patients enrolled in April 2000 also had midweek predialysis blood samples drawn for serum folate and vitamin B12 levels. The presence of risk factors for atherosclerosis and history of clinically significant ASVD (ischemic heart disease, cerebrovascular disease, or peripheral vascular disease) was elicited using results of the patient questionnaire and verified by careful inpatient and outpatient chart review. The charts contained records of all results of outpatient cardiovascular testing and previous hospital admissions. All ASVD events occurred and recorded during the hemodialysis period.
Definition of Outcome Variables
Risk factors for atherosclerosis were defined as follows. Hypertension was defined as the requirement for antihypertensive medications or a resting predialysis blood pressure greater than 140 mmHg systolic or greater than 90 mmHg diastolic on three occasions. Diabetes mellitus was defined by the need for oral hypoglycemics, insulin or a diabetic diet or a fasting or random plasma glucose level greater than 140 mg/dL. Family history of atherosclerotic heart disease was considered significant if a first-degree relative had premature ischemic heart disease (man aged <55 years; woman aged <65 years). A patient was considered to have hypercholesterolemia if they were receiving lipid-reducing agents or had documented total hypercholesterolemia 240 mg/dL; low-density lipoprotein 130 mg/dL. A positive smoking history was recorded when an individual was a current smoker or had quit <1 year prior to being interviewed. Clinically significant ASVD was defined by the presence of ischemic heart disease, cerebrovascular disease, or peripheral vascular disease as described next.
Ischemic heart disease was diagnosed when one or more of the following specific criteria were found. Previous admission for documented myocardial infarction (prolonged chest pain associated with electrocardiogram changes and an elevated creatine kinase level). Significant positive results on coronary angiograms (stenosis of 90% for 1 of the 3 major vessels or 75% narrowing of the left main coronary artery) or thallium scan (definite fixed or reversible perfusion defects). Symptoms consistent with angina confirmed by positive exercise stress test results.
Cerebrovascular disease was defined as a previous stroke (acute-onset irreversible neurological deficit such as aphasia, unilateral weakness, etc.) documented by a physician in the patient's medical records. Peripheral vascular disease was defined by previous peripheral vascular surgery, including amputation of ischemic limb.
Laboratory Testing
Plasma total homocysteine concentrations were detected using the fluorescence polarization immunoassay method. Ingestion of food had little effect on homocysteine levels, whereas hemodialysis may decrease levels by 30%. Hence, predialysis nonfasting levels were measured for all patients. Blood samples were collected and spun to plasma within 30 min of collection. The reference range determined in our laboratory for postmenopausal patients with normal coronary angiography results and normal renal function was 5.5 to 15.0 µmol/L. Serum folate and vitamin B12 levels were measured using a SimulTRAC-SNB radioassay kit in our laboratory. Normal serum folate levels for this assay were greater than 1.5 ng/mL with deficiency defined by levels less than 1.5 ng/mL. Normal vitamin B12 levels for this assay were greater than 160 pg/mL, with deficiency defined by levels less than 120 pg/mL.Citation[[13]], Citation[[14]]
Statistical Analyses
All continuous data were tabulated and analyzed as mean ± SEM. Paired and independent t tests were used for within-group and between-group comparisons, respectively. Differences were considered significant at p less than 0.05. Ninety-five percent CIs were used for means and binomial exact 95% CIs were used for proportions.
Multiple logistic regressions were performed using the presence of ASVD as the outcome variable. Predictor variables were the risk factors (age, sex, diabetes mellitus, smoking, and family history of ASVD). Manual backward elimination was then performed by sequentially removing predictor variables when P was greater than 0.10. The prevalence of ASVD among patients grouped according to homocysteine level was compared using multiple logistic regressions, controlling for the effect of diabetes because the prevalence of diabetes was significantly different among these patient groups.
Results
We randomly selected 200 subjects at our hemodialysis center. A total of 196 patients had hyperhomocystinemia (>15) and were enrolled in this study. Baseline patient characteristics and risk factors for atherosclerosis are listed in . Ninety-seven subjects were men and 99 subjects were women. No patient had a vitamin B12 level less than 130 pg/mL. No patient had a serum folate level less than 1.5 ng/mL. The cause of ESRD was diabetes mellitus for 50% of patients, chronic glomerulonephritis for 43%, polycystic kidney disease for 3.5%, systemic lupus erythematous for 1.5%, obstructive uropathy for 1%, and transitional cell carcinoma for 0.5%. Clinically significant ASVD was present in 63 subjects (32.1%). Twenty-one patients had ischemic heart disease, with three cases identified as a result of a documented myocardial infarction, 15 cases identified using angiography or thallium, and three cases identified as a result of angina and positive exercise stress test results. Thirty-five patients had cerebrovascular disease. One patient had peripheral vascular disease. Four patients had ischemic heart disease combined with cerebrovascular disease. One patient had peripheral vascular disease combined with ischemic heart disease.
Table 1. Demographics and risk factors of chronic hemodialysis patients with and without symptomatic ASVD
The mean homocysteine concentration was 29.0 µmol/L (95%CI, 28.1–30.8) overall. The mean serum folate and vitamin B12 levels were 9.9 ng/mL (95%CI, 9.0–10.8) and 776.3 pg/mL (95%CI, 705.9–845.5), respectively. The mean homocysteine concentration was greater (31.9 µmol/L; 95%CI, 29.1–34.7) for patients with clinically significant ASVD than for those without ASVD (28.7 µmol/L; 95%CI, 27.2–30.2; P = 0.04). The mean serum folate level was less (8.8 ng/mL; 95%CI, 7.2–10.5) for patients with clinically significant ASVD than for those without ASVD (10.2 ng/mL; 95%CI, 9.1–11.3; P = 0.20). The mean vitamin B12 level was less (632.1 pg/mL; 95%CI, 530.5–733.8) for patients with clinically significant ASVD than for those without ASVD (822.3 pg/mL; 95%CI, 737.1–907.5; P = 0.02).
Plasma homocysteine concentrations showed negative correlations with serum folate (p = 0.06; ) and vitamin B12 concentrations (p = 0.07; ) in chronic hemodialysis patients with ASVD. Plasma homocysteine concentrations were inversely proportional to serum folate levels (p<0.001; ) in chronic hemodialysis patients without ASVD. There was a trend toward an inverse correlation with vitamin B12 levels (p = 0.44; ) that was not statistically significant when controlled for serum folate levels. There were significant differences between the two groups according to age, homocysteine levels, vitamin B12 levels and glucose intolerance (P<0.05; ).
Figure 1A. The relationship between homocysteine and serum folate levels y = −4.830ln(x) + 39.422, r = −0.308 (P = 0.014) in chronic hemodialysis patients with ASVD.
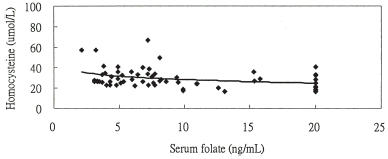
Figure 1B. The relationship between homocysteine and vitamin B12 levels y = −5.228ln(x) + 62.838, r = −0.303 (P = 0.016) in chronic hemodialysis patients with ASVD.
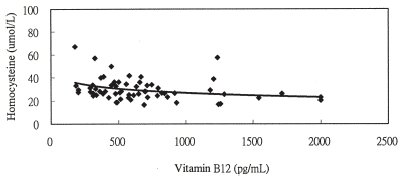
Figure 2A. The relationship between homocysteine and serum folate levels y = −5.819ln(x) + 41.522, r = −0.432 (P<0.001) in chronic hemodialysis patients without ASVD.
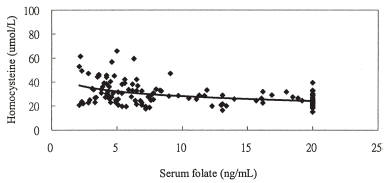
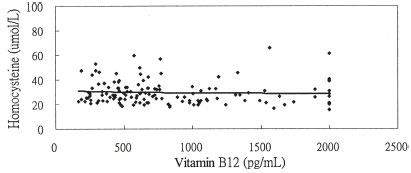
Figure 2B. The relationship between homocysteine and vitamin B12 levels y = −0.856ln(x) + 34.963, r = −0.057 (P = 0.513) in chronic hemodialysis patients without ASVD.
Multiple logistic regression using ASVD as the outcome variable and homocysteine level (as a continuous variable), sex, age, diabetes, smoking, and family history of ASVD as predictor variables showed that for each 10-µmol/L increase in homocysteine level, the odds of having atherosclerotic disease increased by 1.03(P = 0.05). The effect of homocysteine was pronounced at greater concentrations, shown by the relationship between ASVD and homocysteine level adjusted for the effect of diabetes (P = 0.01; ). The unadjusted odds ratio for ASVD was 2.2 (95%CI, 1.06–4.38) for those patients with homocysteine levels in the top quartile compared with those patients with levels in the bottom 3 quartiles. Backward multiple logistic regression was performed using the presence of clinically significant ASVD as the outcome variable () and this identified 6 risk factors (homocysteine value in the highest quartile, sex, age, diabetes, smoking, and family history of ASVD) independently associated with atherosclerotic disease. When adjusted for the effect of sex, age, diabetes, smoking, and family history, the odds of ASVD disease were 2.8-fold greater (95%CI, 1.11–7.47) for patients with homocysteine levels in the top quartile compared with the bottom 3 quartiles.
Figure 3. The prevalence of ASVD by plasma homocysteine level after controlling for the effect of diabetes mellitus (P = 0.01).
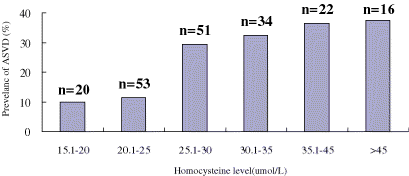
Table 2. Final multiple logistic regression model using ASVD as the outcome variable after manual backward stepwise elimination
Discussion
Hyperhomocystinemia is a common finding in dialysis patients. The prevalence of hyperhomocystinemia in dialysis patients is around 85–100%.Citation[[5]], Citation[[10]], Citation[[11]], Citation[[15]] In our study, the prevalence of hyperhomocystinemia was 98% among hemodialysis patients. The homocysteine level was less than 70 µmol/L in all patients, lower than previously reported.Citation[[16]] It could be attributed to western major diet e.g., beef. Total homocysteine values averaged 29.5 µmol/L which were similar to results previously reported.Citation[[10]] ESRD patients undergoing maintenance hemodialysis experienced high morbidity and mortality rates due to ASVD.Citation[[6]] In our study, ASVD was present in 24.5% (48/148) of patients which was lower than previously reported (45.9%).Citation[[8]] A history of ischemic heart disease was seen in 7.7% (15/196) of patients undergoing hemodialysis, whereas 14.8% (29/196) had documented history of cerebrovascular disease.
The mean homocysteine level in our study population (29.0 µmol/L) was elevated approximately 3-fold greater than the usual level found in other studies of patients with normal renal function.Citation[[1]], Citation[[6]], Citation[[15]], Citation[[17]] This is despite supplementation of these patients in this dialysis unit with an average of 0.71 mg of folic acid and 1 mg of vitamin B12 daily.
Atherosclerotic cardiovascular disease was the cause of death for 25–60% of patients with ESRD and also is major cause of chronic morbidity.Citation[[18]], Citation[[19]] Patients with ASVD had higher hyperhomocystinemia than patients without ASVD.Citation[[8]] In our study, there were associations to homocysteine levels between patients with ASVD and patients without ASVD, which was similar to results of previous reports.
There was association between homocysteine levels and serum folate levels and/or vitamin B12 levels in a majority of the patients, which was similar to results of previous reports.Citation[[20]] Folate depletion was found in the past.Citation[[21]] High-flux hemodialysis may further decrease serum concentrations of hydrosoluble vitamins.Citation[[22]] In our study, only 15% of the patients had folic acid supplementation, but no patient had folic acid deficiency. Ninety-eight percent of the patients had vitamin B complex supplementation, but no patient had also vitamin B12 deficiency. Thirty-five percent of patients had high-flux hemodialysis. All patients did not check serum vitamin B6 level.
In our study, the relationship between homocysteine and atherosclerosis has been preformed and follow up for an average of 18 months. Patients with homocysteine levels in the top quartile compared with the lower 3 quartiles had a relative of 2.8 (95% CI, 1.109–7.467) for developing atherosclerotic event. Moustapha et al.Citation[[7]] reported a prospective study with 167 patients undergoing dialysis, whose results were followed up for an average of 17 months. They showed patients in the upper quartile of homocysteine values were more likely to experience a cardiovascular complication or death from a cardiac cause.
In conclusion, the prevalence of hyperhomocystinemia is 98% among hemodialysis patients. There is a significant association between hyperhomocystinemia and ASVD in Taiwanese chronic hemodialysis patients. There are also associations between homocysteine levels and serum folate/vitamin B12 levels in the majority of the patients. Clinical trials are needed to determine the optimal therapy of folic acid dose for hyperhomocystinemia in Taiwanese chronic hemodialysis patients in the future.
References
- Chauveau P., Chadefaux B., Coude M., Aupettt J., Hannedouche T., Kamoun P., Jungers P. Hyperhomocystinemia, a risk factor for atherosclerosis in chronic uremic patients. Kidney Int. 1993; 41(suppl)S72–S77
- Arnadottir M., Brattstrom L., Simonsen O., Thysell H., Hultbero B., Andersson A., Nilsson-Ehle P. The effect of high-dose pyridoxine and folic acid supplementation on serum lipid and plasma homocysteine concentrations in dialysis patients. Clin. Nephrol. 1993; 40: 236–240
- Bachmann J., Tepel M., Raidt H., Riezler R., Graefe U., Langer K., Zidek W. Hyperhomocystinemia and the risk for vascular disease in hemodialysis patients. J. Am. Soc. Nephrol. 1995; 6: 121–125
- Bostom A.G., Shemin D., Lapane K.L., Miller J.W., Sutherland P., Nadeau M., Seyoum E., Hartman W., Prior R., Wilson P.W.F., Selhub J. Hyperhomocystinemia and traditional cardiovascular disease risk factors in end-stage renal disease patients on dialysis: a case-control study. Atherosclerosis 1995; 114: 93–103
- Robinson K., Gupta A., Dennis V.W., Arheart K., Chaudhary D., Green R., Vigo P., Mayer E.L., Selhub J., Kutner M., Jacobsen D.W. Hyperhomocystinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation 1996; 94: 2743–2748
- Bostom A.G., Lathrop L. Hyperhomocystinemia in end-stage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997; 52: 10–20
- Moustapha A., Naso A., Nahlawi M., Gupta A., Arheart K.L., Jacobsen D.W., Robinson K., Dennis V.W. Prospective study of hyperhomocystinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation 1998; 97: 138–141
- Manns B.J., Burgess E.D., BSc-Hyndman M.E., Parsons H.G., Schaefer J.P., Scott-Douglas N.W. Hyperhomocystinemia and the prevalence of atherosclerotic vascular disease in patients with end-stage renal disease. Am. J. Kid. Dis. 1999; 34: 669–677
- Manns B.J., Burgress E.D., Parsons H.G., Schaefer J.P., Hyndman M.E., Scott-Douglas N.W. Hyperhomocystinemia, anticardiolipin antibody status, and risk for vascular access thrombosis in hemodialysis patients. Kidney Int. 1999; 55: 315–320
- Moustapha A., Gupta A., Robinson K., Arheart K., Jacobsen D.W., Schreiber M.J., Dennis V.W. Prevalence and determinants of hyperhomocystinemia in hemodialysis and peritoneal dialysis. Kidney Int. 1999; 55: 1470–1475
- Bostom A.G., Shemin D., Verhoef P., Nadeau M.R., Jacques P.F., Selhub J., Dworkin L., Rosenberg I.H. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients: a prospective study. American Heart Association, Inc. 1997; 69: 2554–2558
- Bostom A., Brosnan J.T., Hall B., Nadeau M.R., Selhub J. Net uptake of plasma homocysteine by the rat kidney in vivo. Atherosclerosis 1995; 116: 59–62
- Jacob R.A., Wu M.M., Henning S.M., Swendseid M.E. Homocysteine increases as folate decreases in plasma of healthy men during short-term dietary folate and methyl group restriction. J. Nutr. 1994; 124: 1072–1080
- Ubbink J.B., Hayward V.W.J., van der Merwe A., Beeker P.J. Vitamin B12, vitamin B6, and folate nutritional status in men with hyperhomocystinemia. Am. J. Clin. Nutr. 1993; 57: 47–53
- Tremblay R., Bonnardeaux A., Gradah D., Busque L., Lebrun M., Ouimet D., Leblanc M. Hyperhomocystinemia in hemodialysis patients: effect of 12-month supplementation with hydrosoluble vitamins. Kidney Int. 2000; 58: 851–858
- Hultberg B., Andersson A., Sterner G. Plasma homocysteine in renal failure. Clin. Nephrol. 1993; 41: 230–234
- Bostom A., Lathrop L. Hyperhomocystinemia in end-stage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997; 52: 10–20
- Green D., Stone N.J., Krumlovsky F.A. Putative atherogenic factors in patients with chronic renal failure. Prog. Cardiovasc. Dis. 1983; 26: 133–140
- Tamura T., Bergman S.M., Morgan S.L., Homocysteine B. vitamins, and vascular-access thrombosis in patients treated with hemodialysis. Am. J. Kid. Dis. 1998; 32: 475–481
- Manns B., Hyndman E., Burgess E., Parsons H., Schaefer J., Snyder F., Scott-Douglas N. Oral vitamin B12 and high-dose folic acid in hemodialysis patients with hyper-homocyst (e) inemia. Kidney Int. 2001; 59: 1103–1109
- Lasseur C., Parrot F., Delmas Y., Level C., Ged C., Redonnet-Vernhet I., Montaudon D., Combe C., Chauveau P. Impact of high-flux/high-efficiency dialysis on folate and homocysteine metabolism. Journal of Nephrology 2001; 14: 32–35
- Chandna S.M., Tattersall J.E., Nevett G., Tew C.J., O'sullivan J., Greenwood R.N., Farrington K. Low serum vitamin B12 levels in chronic high-flux hemodialysis patients. Nephron 1997; 75: 259–263
