Abstract
Renal failure is a frequent event after cardiopulmonary by-pass. Hemodynamic alterations that occur during surgery, as well as factors depending on the host, are the main risk factors for renal dysfunction. To evaluate the frequency and risk factors for renal dysfunction in this setting, a cohort of fifty patients with preoperative serum creatinine under 1.5 mg/dL, submitted to cardiac surgery with cardiopulmonary by-pass was analyzed. Variables related to preoperative patient condition, intraoperative and postoperative periods were recorded. Renal function was assessed by clearances of creatinine, urea and free water, also by fractional excretion of sodium (FENa), at baseline, at anesthetic induction and during postoperative period. Patients were arbitrarily divided in two groups, according to the serum creatinine (SCr) value at the end of the postoperative period: Group I: SCr <2 mg/dL (n = 44 patients (88.5%)) and Group II: SCr >2 mg/dL (n = 6 patients (11.5%)). A decrease of renal function was observed in all patients: creatinemia raised from 1.04 ± 0.2 to 1.55 ± 0.4 mg/dL (33%), associated with a rise in FENa. Differences between group I and group II using univariate analysis were: baseline serum creatinine (1.01 ± 0.23 mg/dL vs. 1.26 ± 0.19 mg/dL, p = 0.03), FENa (0.99 ± 0.8 vs. 2.2 ± 2.1, p = 0.04), furosemide dose during surgery normalized to body surface area (93.2 ± 23 mg/1.73 m2 BSA vs. 135 ± 38 mg/1.73 m2 BSA, p<0.001), and hemodilution index (17.3 ± 4.3% vs. 22.8 ± 3.2%, p<0.01). In the multiple regression model, baseline creatinemia and furosemide dose were associated to renal dysfunction.
Introduction
Major surgery is a well-established risk factor for development of acute renal failure (ARF).Citation[[1]], Citation[[2]], Citation[[3]]
Open-heart surgery adds supplementary risk because of the severe hemodynamic alterations that occur during cardiopulmonary by-pass (CPB).Citation[[4]] Frequency of ARF in this setting is variable, ranging from 1.1–5% for severe forms requiring renal replacement therapy,Citation[[4]], Citation[[5]] up to 21% in less severe forms.Citation[[2]] Moreover, according to some investigators, a transient decline in renal function is a regular consequence of extracorporeal circulation.Citation[[6]]
ARF after cardiac surgery is associated with a dramatic rise in mortality rate. In cases of severe renal insufficiency, fatality reaches to 31–90%Citation[[7]], Citation[[8]] and in mild forms, to 10–20%.Citation[[2]] In addition to mortality, ARF adds morbidityCitation[[2]] and increase the length of stay and hospital charges.Citation[[8]], Citation[[9]], Citation[[10]]
Renal injury depends mainly on hemodynamic alterations that may occur along surgery and the postoperative period. Among these alterations the following factors are included: non pulsatile flux during the CPB, low mean arterial pressure (MAP) usually below the renal autoregulation level,Citation[[11]] bio-incompatibility mediated by the extracorporeal perfusion system,Citation[[12]] hemolysis, hypothermia and low cardiac output secondary to myocardial depression,Citation[[13]] potentially deleterious effects of vasoactive and inotropic drugs and the use of nephrotoxic drugs, as well as the existence of previous nephropathy or chronic renal failure.
This study describes the changes that occur in renal function after cardiac surgery with cardiopulmonary by-pass and establishes risk factors for the development of ARF in patients with preoperative normal renal function.
Methods
Fifty adult patients submitted to cardiac surgery with total cardiopulmonary by-pass, in Instituto Nacional de Cirugía Cardíaca—IMPASA between 1st March to 31st May 1998 with preoperative serum creatinine (SCr) level less or equal to 1.50 mg/dL were included.
Anesthetic and Surgical Technique
Anesthesia was performed as usual (diazepam 0.3 mg/kg and morphine 0.5 mg/kg at induction continued by isoflurane 1–2% inhalator anesthetics on maintenance); pancuronium was employed for curarization. Mechanical ventilation during surgery was performed with a volumetric respirator (Engström®). EKG was continuously monitored in DII derivation. Systolic, diastolic, and mean arterial pressures were registered through radial artery cannulation. The central venous pressure (CVP) was monitored through subclavian or internal jugular vein catheterization. A bladder catheter was placed for diuresis control and to collect urine along the study period.
Sodium heparin was used for anticoagulation at an initial dose of 300 UI/kg, supplementary doses were administered to allow an activated coagulation time higher than 480 sec. At surgery end, protamine sulfate was administered in a dose sufficient to reach an activated coagulation time between 90 and 120 s.
Cardiopulmonary by-pass was performed in all cases, with a non-pulsatile regimen. The system was primed with crystalloid solution, plus sodium bicarbonate and mannitol 20%. The systemic flux during CPB was maintained close to 2.2 L/min/m2 body surface area and MAP nearby 60 ± 10 mmHg. Membrane oxygenators were used in all cases. All patients were operated in mild systemic hypothermia (31–34°C). Mannitol and furosemide were used at 3 mL/kg and 1 mg/kg respectively, at the beginning of the CPB. Supplementary doses of furosemide were administered if diuresis on surgery was inappropriate to volume status or hemodilution.
Cefuroxime was used for antibiotic prophylaxis.
The following variables were prospectively recorded:
Demographics and preoperative variables. Age, gender, height, weight, body surface area, comorbidities (diabetes, hypertension, nephropathy), underlying cardiac disease, drug exposure (radiocontrast, nonsteroids anti-inflammatory drugs, β-blockers, calcium antagonists, angiotensin-converting enzyme inhibitors, nephrotoxic antibiotics).
Intraoperative variables. Type of surgery (aortocoronary by-pass, valve surgery, combined surgery, aortic artery surgery, other), cardiopulmonary by-pass time (CPBT), aortic cross-clamping time (ACT), MAP on perfusion time and aortic cross-clamping time, diuresis, furosemide dose, mannitol dose, hemodilution index (preoperative Hct—Hct on CPB),Citation[[7]] autotransfusion volume, inotropic drugs, intra-aortic counterpulsation balloon use. A low cardiac output was considered when more than 8 µg/kg/min dopamine or dobutamine, and/or adrenaline, noradrenaline or isoproterenol at any dose were needed to stabilize hemodynamic status.Citation[[8]] Intra-aortic counterpulsation balloon was placed when drugs failed.
Postoperative variables. MAP, CVP, average MAP and CVP six hours previous to the maximum SCr level reached, diuresis, inotropic drugs use, ICU length stay, complications other than ARF, in-hospital mortality.
Assessment of renal function. Urine and serum levels of urea, creatinine, sodium, potassium, chloride, and osmolality were measured. Blood and urine samples were taken at anesthetic induction (T0) and during the postoperative period at the first hour (T1), 6 h (T2), 12 h (T3), and 24 h (T4). Urine output was measured at the same periods. Creatinine, urea, osmolar and free water clearances, fractional excretion of sodium, potassium and chloride, and trans tubular potassium gradient were then calculated.
The Institutional Review Board approved the protocol.
Statistical Analysis
Data are expressed as mean ± SD, or median and range. For univariate analysis, Student's “t” test, ANOVA, Mann-Whitney or Wilcoxon rank tests were used for continuous variables. Chi-square test or exact Fisher test for qualitative variables. Multiple regression analysis and bivariate logistic regression model were used to identify independent risk factors for renal dysfunction. Longitudinal data were analyzed with repeated measures ANOVA or Kruskall-Wallys test. A probability less than 5% for the null hypothesis was considered of statistic significance. All tests were two-tailed. Statistical package SPSS 9.0 (SPSS Inc., Chicago, Illinois) was used for data processing and statistical analysis.
Results
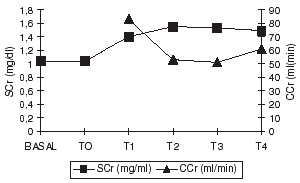
All 50 patients stayed along the study period. Thirty-two (64%) were male and eighteen (36%) female. Mean age was 61.4 ± 9.9 y (30–80 y). Forty-six patients had comorbidities: hypertension (36), diabetes (12), and nephropathy (2). Underlying cardiac disease was ischemic in the majority of cases (38) and valvular disease (10). Thirty-two patients were treated with antiplatelet drugs prior to surgery, twenty-three received β-blockers, fifteen ACEI, and fourteen, calcium antagonist. In patients exposed to radiocontrast agents, the median time between exposition and surgery was 1 day (range: 1–15 days). Tables and summarize intraoperative variables. Aorto-coronary by-pass was the predominant type of surgery. Mean CPBT was 77.7 ± 34 min (median: 71.5 min; range: 22–215 min), ACT was 42.9 ± 19.8 min (median: 37.5 min; range: 8–101 min). Mean mannitol dose 61.5 ± 21.3 g and mean furosemide dose was 101.5 ± 28 mg. MAP values during intraoperative and postoperative period are shown in .
Table 1. Intraoperative variables
Table 2. Values of MAPFootnotea during surgery
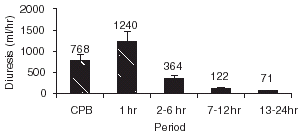
provides information on SCr and creatinine clearance. There was an early decline of renal function after open-heart cardiac surgery, but in none of the patients renal failure was mild or severe. Serun creatinine increased from baseline normal values in all patients in the first 6 h PO (1.03 ± 0.28–1.53 ± 0.4 mg/dL), with a progressive decline to 1.46 ± 0.48 mg/dL at 24 h PO. Creatinine clearance showed a marked decline from preoperative values in all patients, with a mean percentage reduction of 28.2 ± 16.5% (p<0.001) at 24 h PO. There was a frank polyuria in the first hour PO (1240 mL/h), with a progressive decline of urine output to normal values after 12 h PO (71 mL/h) (). Diuresis was not correlated with SCr, CCr, or furosemide dose.
Figure 1. Serum creatinine (SCr) (mg/dl), and creatinine clearance (CCr) (mL/min) through the observation period.
For the analysis of risk factor for ARF, patients were arbitrarily divided into two groups according to SCr at the end of the observation period (T4): group I: SCr <2 mg/dL, (46 patients (88.5%)); group II: SCr ≥2 mg/dL (six cases (11.5%)). None of the patients required renal replacement therapies.
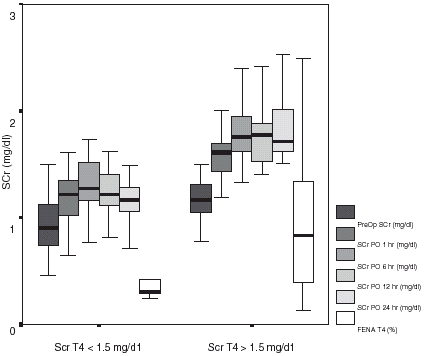
shows SCr evolution in Group I and Group II patients. Group I patients had a rise in SCr with a concomitant decline in CCr in the first 6 h PO, with partial recovery at 24 h PO. Serum creatinine in Group II continued to rise at 24 h PO. FeNa was within normal values at 24 h PO in Group I while continued elevated in Group II patients (1.3 ± 0.3% vs. 2.4 ± 0.4%; p<0.05) (). In 20 cases (40%), SCr remained above 1.5 mg/dL at the end of the observation period (). All but six patients (88%) continued with a depressed creatinine clearance despite of “normal” creatinine values at 24 h postoperative.
Figure 3. Evolution of serum creatinine (SCr) along the observation period in patients with SCr at T4 less or equal to 2 mg/dl (Group I), or above 2 mg/dl (Group II). It is also shown FENa at the end of the study (T4).
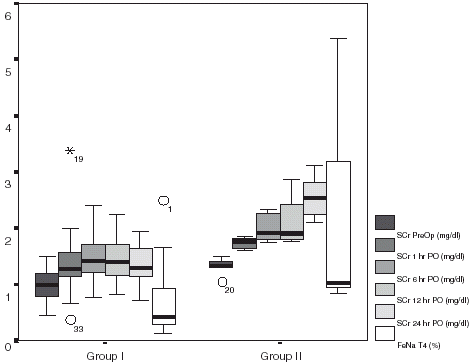
Figure 4. Evolution of serum creatinine (SCr) along the observation period in patients with SCr at T4 less or equal to 1.5 mg/dL, or above 1.5 mg/dL. It is also shown FENa at the end of the study (T4).
In Tables and are showed results of univariate and multivariate analysis, respectively. There were no differences between preoperative or intraoperative hemodynamic variables between groups. Also, there were no differences between group in urea, osmolar and free water clearance, potassium, chloride, and transtubular potassium gradient.
Table 3. Results of univariate analysis. Only baseline SCr, FENa at T4, frusemide dose and hemodilution were statistically associated with groups
Table 4. In multivariate analysis, only baseline Scr and furosemide dose reached statistical significance
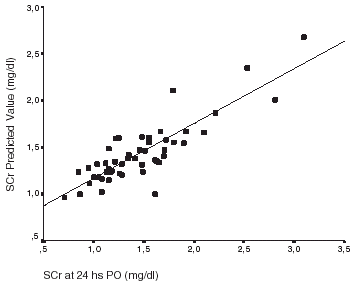
In univariate analysis baseline SCr, FENa at T4, furosemide dose, and hemodilution index were significantly different between groups (). In multivariate logistic regression analysis only baseline SCr (GI: 1.01 ± 0.23 mg/dL, GII: 1.26 ± 0.19 mg/dL, p = 0.03) and intraoperative furosemide dose (GI: 93.3 ± 23 mg/1.73 m2 BSA; GII 135 ± 38.4 mg/1.73 m2 BSA, p<0.01) were independently associated to renal dysfunction. Also, in multiple regression analysis, variables significantly associated with SCr at 24 h. were furosemide dose (β = 0.707) and baseline creatinine (β = 0.474) (). Observed and calculated SCr at 24 h showed a high correlation (r = 0.836, p<0.05). ().
Discussion
Acute renal failure is a frequent complication in open-heart surgery. Severe ARF requiring renal replacement therapy is seen in 2–5% of cases,Citation[[4]], Citation[[5]], Citation[[8]] but less serious forms are even more frequent.Citation[[5]]
In the present study, all population showed a decrease in renal function, according to SCr and CCr. On the other hand, FENa presented the expected changes at the beginning of postoperative period due to diuretic use, but kept on being elevated until the end of the observational period only in Group II. In our opinion, this finding must be considered as an evidence of tubular injury in this subset of patients.
It must be remarked that renal dysfunction was detected early, at the first hour of postoperative period and was the greatest decline at six hour after surgery. At the end of the 24-h period six patients (11.5%) had SCr higher than 2 mg/dL, 20 patients (40%) had SCr higher than 1.5 mg/dL, but it must be noted that all but six patients continued with a depressed creatinine clearance despite of “normal” creatinine values at 24 h postoperative. Usually SCr is determined in cardiac surgery patients after 24 h of surgery, so early and transient renal dysfunction can be overlooked. Our data are in accordance with those of Wesslink et al.Citation[[6]] who pointed out that renal dysfunction is a result, almost unavoidable of extracorporeal circulation.
Cardiopulmonary by-pass determine negative effects on renal circulation, as a consequence of nonpulsatile flux, decrease of renal perfusion pressure under the autoregulation level and free hemoglobin toxicity secondary to hemolysis due to extracorporeal circulation.Citation[[14]], Citation[[15]] On the other hand, systemic inflammatory response mediated by cytokines and other mediators, stimulated by bioincompatible membranes, plus the effect of tissular injury due to surgery, hypothermia, aortic-clamping, and myocardial reperfusionCitation[[12]], Citation[[13]], Citation[[14]], Citation[[16]], Citation[[17]] lead to hemodynamic changes and endothelial damageCitation[[18]], Citation[[19]], Citation[[20]], Citation[[21]] which are in the base of renal injury.
We have not been able to demonstrate any association among variables related to cardiopulmonary by-pass and renal dysfunction, as almostCitation[[6]], Citation[[13]], Citation[[22]] but not allCitation[[8]], Citation[[11]] authors did. In a case-control study previously performed in our institution,Citation[[10]] an association between cardiopulmonary by-pass time and renal failure was found, but it must be pointed out that CPB time in the past was longer than in the present study. Similar considerations can be done regarding intraoperative MAP, which commonly reached lower levels than nowadays.Citation[[11]]
We have neither found association with postoperative hemodynamic variables, nor the use of vasoactive drugs. Exposition to nephrotoxic drugs, particularly radio-contrast media, showed no association with renal dysfunction.
In our opinion, the lack of association between hemodynamic variables and renal dysfunction is relevant. One can hypothesize that progress in surgery and anesthetic techniques, particularly reduction of operatory time, improvement in cardiopulmonary by-pass equipment, use of more biocompatible membranes and improvement on hemodynamic support during and after surgery, have reduced the risk of renal injury. On the other hand, those factors related to the host, little or not modifiable, could play a leading role in the pathogenesis of renal failure. Percutaneous transluminal coronary angioplasty had selected patients leaving to surgery the higher risk patients, according to the complexity and severity of illness.
In the present study, renal dysfunction at 24 h after surgery was clearly related with baseline serum creatinine: SCr of Group II was higher than SCr of Group I (1.26 ± 0.13 mg/dL vs. 1.01 ± 0.23 mg/dL, p = 0.03). It must be pointed out that this variable was predictive of renal dysfunction, in spite of being near normal values. This predictive ability persists even if the arbitrary SCr value used to define groups is reduced from 2 mg/dL to 1.5 mg/dL: 0.92 ± 0.27 mg/dL vs 1.17 ± 0.21 mg/dL; p<0.01 (data not shown in results). In a retrospective study of 43,642 patients who underwent cardiac surgery with cardiopulmonary by-pass, Chertow et al.Citation[[5]] found that a preoperative SCr higher than 1.5 mg/dL was a risk factor for the development of renal failure. In the present group of patients, predictive SCr level was as lower as 1.26 mg/dL. A number of investigators have shown that previous renal disease predispose to postoperatory acute renal failure.Citation[[2]], Citation[[4]], Citation[[5]], Citation[[8]] It is possible to hypothesize that serum creatinine, even normal, undervalue the preoperatory renal function in this setting (advanced age, malnutrition, hypertension, diabetes).
Group II patients received a higher dose of furosemide than Group I patients (Group I = 96.5 ± 20.3 vs. Group II = 140 ± 49 mg, p = 0.001). As mentioned above, furosemide was routinely used during the operative time at a previously defined bolus administered dose of 1 mg/kg, but supplementary doses were added if needed. So, individual doses ranged between 40 and 200 mg. There was a lack of correlation between furosemide dose and IO or PO diuresis, so there was no dose-response correlation. Francis et al.Citation[[23]] found that administration of furosemide in bolus had vasoconstrictive effect by activation of neuro-endocrine axis, in patients with cardiac failure. Yetman and co-workersCitation[[24]] described, in pediatric patients, that furosemide (1 mg/k body weight, i/v) led to fall in cardiac index, rise in systemic vascular resistance and increase in ranine and norepinephrine levels. It is conceivable that these effects of furosemide may reinforce adverse hemodynamic consequences of cardiopulmonary by-pass, magnifying misdistribution of renal blood flow and sectorial renal hypoperfusion.Citation[[25]], Citation[[26]] Furosemide is also able to determine direct tubular toxicity, which is associated with evidence of tubular dysfunction. A RCT performed to test the preventive effect of furosemide in patients submitted to radiocontrast studies, demonstrated a deleterious more than protective effect of furosemide in this setting.Citation[[27]] To our knowledge, no previous studies reported any adverse effect of furosemide in cardiac surgery patients.
Hemodilution index was higher in Group II patients (22.8 ± 3.2 vs. 17.3 ± 4.3, p = 0.004). These findings are in opposition with those referred by other investigators. Slogoff et al.Citation[[11]] found that the use of crystalloids depress blood viscosity, improving intrarenal blood flow. Myers et al.Citation[[13]] arrived to similar conclusions. On the other hand, Hardy and co-workersCitation[[28]] showed that mortality rate was higher if hemoglobin level attain 5 g/dL or less; lactacidemia was elevated in these cases, therefore one may hypothesize that renal hypoxia could co-exist. Preoperative renal disfunction, renal hypoperfusion and hemodilution would be deleterious to renal function in CPB cardiac surgery, associated with a vasoconstrictive effect of bolus intraoperative administered furosemide. In the multivariate logistic regression analysis only preoperative serum creatinine and intraoperative furosemide dose were significantly associated with depressed renal function at 24 h.
In summary: the present study demonstrates an early decline of renal function after open-heart cardiac surgery, but in none of the patients renal failure was mild or severe. In 20 cases (40%), renal function remained abnormal at the end of the observation period, associated with evidence of tubular impairment in those patients with Scr higher than 2 mg/dL at 24 h from surgery. Baseline serum creatinine level, in spite of being within normal values, was highly predictive of postoperative renal dysfunction, which in our opinion, confirm that creatinemia underestimate renal reserve in this setting. All but six patients (88%) continued with a depressed creatinine clearance despite of “normal” creatinine values at 24 h postoperative. Intraoperative dose of furosemide was associated with renal dysfunction, probably due to an increase on renal vascular resistance or direct tubular nephrotoxicity induced by the diuretic. So, in the lack of evidence of the benefit of furosemide as a protector drug in ARF, it should be avoided in cardiac surgery in the doses administered in the present study.
References
- Nolan C.R., Anderson R.J. Hospital-acquired acute renal failure. J. Am. Soc. Nephrol. 1998; 9: 710–718
- Corwin H.L., Sprague S.M., DeLaria G.A., Norusis M.J. Acute renal failure associated with cardiac operations. J. Thorac. Cardiovasc. Surg. 1989; 8: 1107–1112
- Morris Davidman, Paul Olson, Jeffrey Kohen, Tom Leither, Carl Kjelstrand. Iatrogenic renal disease. Arch. Intern. Med. 1991; 151: 1809–1812
- Kellerman P.S. Perioperative care of the renal patient. Review article. Arch. Int. Med. 1994; 154: 1674–1688
- Chertow G.M., Lazarus J.M., Christiansen C.L., Cook E.F., Hammersmeister K.E., Grover F., Daley J. Preoperative renal risk stratification. Circulation 1997; 95: 878–884
- Wesselink R.M.J., De Boer A., Morshuis W.J., Leusink J.A. Cardio-pulmonary-bypass time has important independent influence on mortality and morbidity. Eur. J. Cardiothorac. Surg. 1997; 11: 1141–1145
- Mondero P., Sáliz N., Panadero A. Protección renal perioperatoria: bases fisiopatológicas de la insuficiencia renal aguda y medidas profilácticas. Rev. Esp. Anestesiol. Reanim. (Spanish) 1998; 45: 50–63
- Zanardo G., Michielon P., Paccagnella A., Rossi P., Caló M., Salandin V., Da Ros A., a Michieletto F., Simini G. Acute renal failure in the patient undergoing cardiac operation. J. Thorac. Cardiovasc. Surg. 1994; 107: 1489–1495
- Smith P.K., Smith R., Muhlbaier L.H. Risk stratification for adverse economic outcomes in cardiac surgery. Ann. Thorac. Surg. 1997; 64(S)61–63
- Llopart T., Lombardi R., Forselledo M., Andrade R. Acute renal failure in open-heart surgery. Renal Failure 1997; 9: 319–323
- Slogoff S., Reul G.J., Keats A.S., Curry G.R., Crum M.E., Elmquist B.A., Giesecke M.N., Jistel J.R., Rogers L.K., Soderberg J.D., Edelman S.K. Role of perfusion pressure and flow in major organ dysfunction after cardiopulmonary bypass. Ann. Thorac. Surg. 1990; 50: 911–918
- Edmunds L.H. Inflammatory response to cardiopulmonary bypass. Ann. Thorac. Surg. 1998; 66(S)12–16
- Myers B.D. Nature of postischemic renal injury following aortic or cardiac surgery. Acute Renal Failure in the Intensive Therapy Unit, D. Bihari, G. Neild. Springer-Verlag, London 1990; 167–180
- Kron I.L., Joob A.W., Van Meter C. Acute renal failure in the cardiovascular surgical patient. Review. Ann. Thorac. Surg. 1985; 39: 590–598
- Lodge A.J., Ündar A., Daggett W., Runge T.M., Calhoom J.H., Ungerleider R.M. Regional blood flow during pulsatile cardiopulmonary by-pass and circulatory arrest in an infant model. Ann. Thorac. Surg. 1997; 63: 1243–1250
- O'Dwyer C., Woodson L.C., Conroy B.P., Lin C.Y., Deyo D.J., Uchida T., Johnston W.E. Regional perfusion abnormalities with phenylephrine during normothermic by-pass. Ann. Thorac. Surg. 1997; 63: 728–735
- Ávila F.J., Amador-Amerigo I., Rodriguez-Mateos P. Síndrome postperfusión del by-pass cardiopulmonar. Revisión monográfica. Cir Cardiov (Spanish) 1996; 3: 1–33
- Verrier E.D., Morgan E.N. Endothelial response to cardiopulmonary bypass surgery. Ann. Thorac. Surg. 1998; 66(S)17–19
- Verrier E.D., Boyle E.M. Endothelial cell injury in cardiovascular surgery. Ann. Thorac. Surg. 1996; 62: 915–922
- Boyle E.M., Pohlman T.H., Cornejo C.J., Verrier E.D. Endothelial cell injury in cardiovascular surgery: ischemia-reperfusion. Ann. Thorac. Surg. 1996; 62: 1868–1875
- Gott J.P., Cooper W.A., Schmidt F.E., Brown W.M., Wright C.E., Merlino J.D., Fortenberry J.D., Clark W.S., Guyton R.A. Modifying risk for extracorporeal circulation: trial of four antiinflammatory strategies. Ann. Thorac. Surg. 1998; 66: 747–754
- Urzua J., Troncoso S., Bugedo G., Canessa R., Muñoz H., Lema G., Valdivieso A., Irarrazaval M., Moran S., Meneses G. Renal function and cardiopulmonary bypass: effect of perfusion pressure. J. Cardiothorac. Vasc. Anesth. 1992; 6: 299–303
- Francis G.S., Siegel R.M., Goldsmith S.R., Olivari M.T., Levine T.B., Cohn J.N. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann. Intern. Med. 1985; 103: 1–6
- Yetman A.T., Singh N.C., Parbtani A., Loft J.A., Linley M.A., Johnson C.C., Morgan D. Acute hemodynamic and neurohormonal effects of furosemide in critically ill pediatric patients. Crit. Care Med. 1996; 24: 398–302
- Lema G. Renal protection. Cardioth. Vasc. Anesth. 1998; 265–270
- Pathi V.L., Morrison J., MacPhaden A., Martin W., McQuiston A.M., Wheatley D.J. Alterations in renal microcirculation during cardiopulmonary bypass. Ann. Thorac. Surg. 1998; 65: 993–998
- Weinstein J.M., Heyman S., Brezies M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron 1992; 62: 413–415
- Hardy J.F., Bélisle S., Janvier G., Samama M. Reduction in requirements for allogeneic blood products: nonpharmacologic methods. Ann. Thorac. Surg. 1996; 62: 1935–194