Abstract
Background: Peripheral neuropathy is considered a common complication in patients suffering from advanced chronic kidney disease (CKD). Superimposed peripheral multiple neuropathies may complicate arteriovenous (A‐V) fistulas construction. Aim: To evaluate, prospectively, the influence of brachiocephalic A‐V fistulas construction on the peripheral nerves of the same extremity and to characterize the patients at risk for developing ischemic and neurological complications. Patients and Methods: Twenty patients suffering from advanced CKD were enrolled in the study: 10 diabetic and 10 non‐diabetic patients. All patients underwent electrophysiological evaluation one week before, 3 weeks and 3 months after surgery. Median, ulnar and radial nerves were studied. Results: In non‐diabetic patients MNCV was normal before and after surgery, but were significantly lower and reduced progressively and significantly after surgery in diabetic patients (p ≤ 0.02). In both non‐diabetic and diabetic patients SNCV was reduced, but were significantly lower in diabetic patients before and after surgery (p ≤ 0.03). In diabetic patients it reduced progressively and significantly after surgery (p < 0.01). Thirty percent of patients developed local edema and significant decrease of CMAP of median nerve three weeks after surgery (p = 0.02) with complete resolution at three months. Conclusion: Diabetic uremic patients are at increased risk to develop disabling neurological complications after the construction of A‐V fistulas. Diabetes was the only predictive risk factor for developing these complications. Prevention requires careful preoperative electrophysiological evaluation and postoperative follow‐up.
Introduction
Peripheral neuropathy is considered a common complication in patients suffering from advanced chronic kidney disease (CKD).Citation[1], Citation[2] At the beginning of dialysis, about two thirds of patients suffer from this complication.Citation[3], Citation[4], Citation[5] All types of chronic kidney disease may be complicated with peripheral neuropathy.Citation[6] Peripheral nerve involvement in CKD can be one of four types. The most common is distal, painless, progressive, symmetrical sensorimotor polyneuropathy evolving slowly over many months.Citation[6] Second, mononeuropathy, including: carpal tunnel syndrome (CTS) in patients receiving long‐term hemodialysisCitation[7], Citation[8], Citation[9], Citation[10], Citation[11] and compression neuropathy.Citation[12] Third, neuropathy resulting from associated systemic diseases, such as diabetes. Fourth, ischemic monomelic neuropathy (IMN) involves multiple distal mononeuropathies occurring in an extremity following the construction of arteriovenous (A‐V) fistula in a proximal major limb artery and usually involving both sensory and motor branches.Citation[3], Citation[13], Citation[14], Citation[15], Citation[16], Citation[17] It occurs predominantly in patients suffering from diabetes mellitus and peripheral vascular disease.Citation[14], Citation[16], Citation[17] In IMN there are more than one major peripheral nerve involvement in contrast to nerve compression neuropathy from hematoma, abscess or aneurysm where a single nerve is involved.Citation[12], Citation[16] The uremic neuropathy is often sub clinical and detectable only by electrophysiological studies.Citation[18], Citation[19], Citation[20], Citation[21] Approximately half of uremic predialytic patients without neurological complaints had motor and sensory peripheral polyneuropathy.Citation[21] Evidence of significant polyneuropathy is seen in 50–60 percent of patients who have been receiving long‐term hemodialysis.Citation[5] Pathologically it is characterized by demyelination and axonal degeneration.Citation[2] The underlying basis of uremic neuropathy is unknown but may be related to accumulation and deposition of toxic substances including methylguanidine and myoinositol.Citation[22], Citation[23] Peripheral multiple neuropathies may complicate A‐V fistulas performed for chronic hemodialysis.Citation[13], Citation[14], Citation[15], Citation[16], Citation[17]
The aim of the study was to evaluate, prospectively, the influence of brachiocephalic A‐V fistulas construction on the peripheral nerves in the same extremity and to characterize the patients at risk for developing ischemic and neurological complications.
Subjects and Methods
Twenty consecutive predialysis patients, ten non‐diabetic and ten diabetic patients were planned for brachiocephalic A‐V fistula construction for starting chronic hemodialysis. The patients included in the study were with creatinine clearance test (CCT) below 20 mL/min, without neurological complaints or evidence of iron, vitamin B12 or folic acid deficiencies. All patients underwent electrophysiological studies after signing an informed consent form, which was approved by the institutional review committee. Nerve conduction studies (NCS) were performed using Nicolet Viking IV instrument before surgery (baseline), at three weeks and at three months after surgery. Motor nerve conduction velocity (MNCV), compound muscle action potentials (CMAP) and motor distal latency (MDL) were recorded in median, ulnar and radial nerves. Sensory nerve conduction velocity (SNCV), sensory nerve action potentials (SNAP) and sensory distal latency (SDL) were recorded in median and ulnar nerves. SNCV were measured by antidromical stimulation of the nerves applied at a frequency of 2 Hz and twenty trials were averaged. All limbs studied were warmed with heat lamp until the skin temperatures recorded were in the range of 33–34°C. The patients were followed every week in the predialysis clinic.
Statistical Analysis
Quantitative variables: age, CCT and electrophysiological parameters (MNCV, SNCV, MDL, SDL, CMAP and SNAP) were described by means and standard deviations. Nonparametric tests were used. Friedman test was used to evaluate the changes in the electrophysiological variables at baseline (before surgery), three weeks and three months after surgery. Mann Whitney test was used to compare these variables between patients who developed local edema and those who did not develop edema and between non‐diabetic and diabetic patients.
Results
The patients' mean age was 59.5 ± 12.7 years (range 26–75), mean CCT 12.5 ± 3.9 mL/min (range 7.5–17.8). The duration of CRF and diabetes mellitus were 5.2 ± 1.1 years (range 2.8–7.3) and 11.0 ± 1.4 years (range 8.3–17.5), respectively.
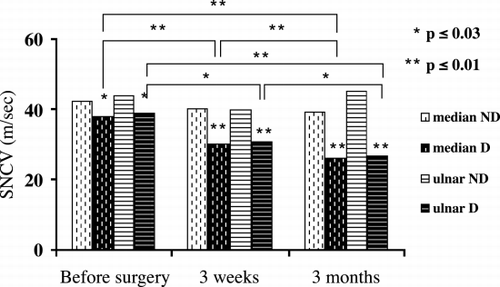
Statistical analysis indicated that that MNCV was within or near normal range before the construction of the brachiocephalic A‐V fistulas. It reduced significantly at three weeks and three months after operation in median and radial nerves (p < 0.01). It also reduced significantly at three months in ulnar nerve (p < 0.01, ). SNCV was reduced in median and ulnar nerves before surgery and decreased progressively and significantly after operation (p < 0.05, ). MDL were within normal range before surgery and rose significantly at three months in all nerves studied (p < 0.01, ). SDL was abnormal before surgery and rose significantly at three weeks and three months in median nerve and at three months after surgery in ulnar nerve (p < 0.01, ). CMAP of median, ulnar and radial nerves and SNAP of median and ulnar nerves were not affected from A‐V fistula surgery.
Table 1. Electrophysiological Results Before and After Brachiocephalic A‐V Fistulas Construction in All Predialysis Patients Enrolled in the Study
In non‐diabetic patients MNCV of median, ulnar and radial nerves were normal before and after surgery, but were significantly lower in diabetic patients (p ≤ 0.02, , ). In diabetic patients, MNCV reduced progressively and significantly after surgery in all nerves studied (p < 0.03, , ). In both non‐diabetic and diabetic patients SNCV of median and ulnar nerves were reduced, but were significantly lower in diabetic patients before and after surgery (p < 0.03, , ). In diabetic patients SNCV reduced progressively and significantly after surgery (p < 0.03, , ). In non‐diabetic patients, MDL were within normal range before surgery and did not change significantly after A‐V fistula procedure in all nerves studied (). In diabetic patients MDL rose significantly after three months and was significantly more elongated in diabetic patients (p ≤ 0.01, ). In both groups, SDL were abnormal before and after surgery. It rose significantly after three months and was significantly more elongated in diabetic patients (p ≤ 0.01, ). CMAP of median, ulnar and radial nerves were normal before surgery, at three weeks and three months in nondiabetic patients but mildly and not significantly reduced in diabetic patients. SNAP of median and ulnar nerves were normal before and after A‐V fistula surgery in nondiabetic patients but were significantly reduced in diabetic patients (). It progressively and significantly decreased after surgery in diabetic patients (p < 0.03, ).
Table 2. MNCV Before and After Brachiocephalic A‐V Fistula Construction in Non‐Diabetic Patients Compared to Diabetic Patients
Table 3. SNCV Before and After Brachiocephalic A‐V Fistulas Construction in Non‐diabetic Patients Compared to Diabetic Patients
Table 4. DML Before and After Brachiocephalic A‐V Fistulas Construction in Non‐diabetic Patients Compared to Diabetic Patients
Table 5. SDL Before and After Brachiocephalic A‐V Fistulas Construction in Non‐diabetic Patients Compared to Diabetic Patients
Table 6. SNAP Before and After Brachiocephalic A‐V Fistulas Construction in Non‐diabetic Patients Compared to Diabetic Patients
Figure 1. MNCV of median, ulnar and radial nerves before surgery (baseline), at three weeks and at three months after the construction of brachiocephalic A‐V fistulas in non‐diabetic (ND) patients compared to diabetic (D) patients.
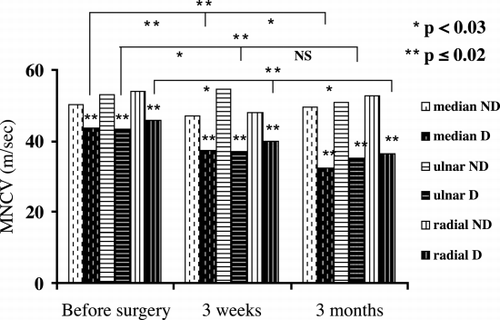
Figure 2. SNCV of median and ulnar nerves before surgery (baseline), at three weeks and at three months after the construction of brachiocephalic A‐V fistulas in non‐diabetic (ND) patients compared to diabetic (D) patients.
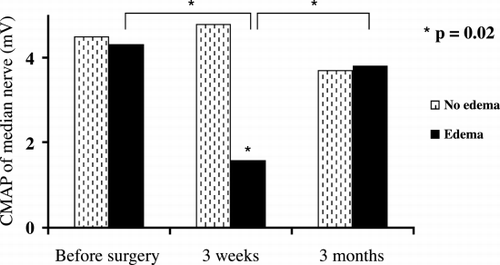
Thirty percent of the patients developed local edema and had a significant decrease of CMAP of median nerve at three weeks after A‐V fistula surgery (p = 0.02) with complete resolution at three months (, ).
Table 7. CMAP Before and After Brachiocephalic A‐V Fistulas Construction of Median Nerve in Patients Who Developed Edema Compared to Patients Who Did Not Develop Edema
Discussion
In patients suffering from advanced CKD, peripheral neuropathy is considered a common complication.Citation[1], Citation[2] Superimposed peripheral multiple neuropathies may complicate the construction of A‐V fistulas for chronic hemodialysis.Citation[13], Citation[14], Citation[15], Citation[16], Citation[17] Our study was aimed to evaluate, prospectively, the influence of the surgical construction of brachiocephalic A‐V fistulas on the peripheral nerves in the same extremity and to characterize the patients at risk for developing ischemic and neurological complications.
Statistical analysis of patients enrolled in the study indicated that MNCV was within or near normal range before surgery but reduced significantly after A‐V fistula construction (p < 0.01, ). SNCV was reduced before surgery and decreased progressively and significantly after operation (p < 0.05, ). MDL were within normal range but SDL was abnormal before surgery. They rose significantly after operation (p < 0.01, ). CMAP and SNAP were not affected from the construction of A‐V fistulas. The reduction in conduction velocities was more pronounced than the reduction in action potentials and was found in all the nerves. These results indicate demyelination, rather than axonal injury and ischemic etiology rather than local compression. The sensory conduction velocities were more affected than the motor velocities. It is well known that uremic and diabetic sensory neuropathy precedes motor neuropathy.Citation[1], Citation[21] Klein et al. evaluated fifteen patients with unilateral functioning A‐V fistulas and reported a reduction in the conduction velocity of the ulnar nerve on the side of the functional fistula.Citation[24]
Some observations indicate that diabetic patients are prone to develop neurological complications more than other patients after the construction of A‐V fistulas.Citation[14], Citation[16], Citation[17] Non‐diabetic and diabetic patients were compared. In non‐diabetic patients MNCV was normal before and after surgery. In diabetic patients, it was reduced, notably lower and decreased progressively and significantly after surgery (p ≤ 0.02, , ). SNCV was reduced in both non‐diabetic and diabetic patients. It was notably lower and decreased progressively and significantly after surgery in diabetic patients (p ≤ 0.03, , ). CMAP was normal in non‐diabetic patients but mildly and not significantly reduced in diabetic patients after operation. SNAP was normal before and after A‐V fistula surgery in non‐diabetic patients (). It was notably reduced and decreased progressively and significantly after operation in diabetic patients (p ≤ 0.03, ). These results demonstrated that diabetic patients are at high risk to develop ischemic and neurological complications compared to non‐diabetic patients after the construction of A‐V fistulas. Diabetic patients are prone to develop diabetic neuropathy and vasculopathy. Therefore, the peripheral nervous system become more affected, especially the sensory parts, when these patients develop uremia. These considerations may explain why SNCV and SNAP were reduced in all nerves studied more than MNCV and CMAP. Redfern et al. reviewed twenty‐two patients on hemodialysis with ischemic or neurological complications.Citation[16] Seventeen of them were diabetic. Twelve patients developed significant motor and/or sensory impairment immediately after surgical construction of the A‐V fistula. Wytrzes et al. evaluated twenty patients with proximal shunts for risk factors for brachial neuropathy.Citation[17] Although all patients had severe atherosclerosis and many had polyneuropathy, they could not identify predictive risk factors other than diabetes. These reports are compatible with our results. We evaluated, prospectively, predialysis patients before and after the surgical construction of the A‐V fistulas. Significant motor and/or sensory impairments were found only in diabetic patients.
Two diabetic patients developed severe symptomatic, ischemic and neurological complications after surgery. Conduction velocities and amplitudes cannot be recorded in all the nerves of the upper extremities on the side of the fistula. Closure of the A‐V fistulas did not improve the neuropathy. Hye et al. reported six episodes of IMN as a complication of upper extremity dialysis grafts.Citation[15] All patients had long‐standing diabetes mellitus. Five episodes occurred immediately after operation. Electrophysiological studies showed underlying diabetic neuropathy with severe multifocal distal neuropathy. In IMN there are more than one major peripheral nerve involvement in contrast to nerve compression neuropathy from hematoma, abscess or aneurysm where a single nerve is involved.Citation[7], Citation[12], Citation[16], Citation[17]
Thirty percent of our patients developed significant decrease of CMAP of median nerve at three weeks after A‐V fistula construction with complete resolution at three months (p = 0.02, , ). Clinically, these patients had local edema that cause transient re versible compression neuropathy and resolved spontaneously at three months.
Although uremic patients had progressive atherosclerosis and polyneuropathy, non‐diabetic patients did not develop significant motor and/or sensory electrophysiological impairments. Diabetic uremic patients are at increased risk to develop disabling neurological complications after the construction of A‐V fistulas. Moreover, diabetes was the only predictive risk factor for developing these complications. Correction of ischemia is indicated but usually does not improve the neuropathy. Prevention requires careful preoperative electrophysiological evaluation and postoperative follow‐up.
References
- Raskin N. H., Fishman R. A. Neurologic disorders in renal failure. N. Engl. J. Med. 1976; 294: 204–210, [PUBMED], [INFOTRIEVE]
- Fraser C. L., Arief A. L. Nervous system complication in uremia. Ann. Intern. Med. 1988; 109: 143–153, [PUBMED], [INFOTRIEVE]
- Robson J. S. Uremic neuropathy. Some Aspects of Neurology, R. F. Robertson. Royal College of Physicians, Edinburgh 1968; 74–84
- Bolton C. F. Peripheral neuropathy associated with chronic renal failure. Can. J. Neurol. Sci. 1980; 7(2)89, [PUBMED], [INFOTRIEVE]
- Asbury A. K. Uremic neuropathy. Peripheral Neuropathy2nd Ed., P. J. Dyck, P. K. Thomas, E. H. Lambert. Saunders, Philadelphia 1984; 1811–1825
- Adams R. D., Victor M. Disease of the peripheral nerves. Principles of Neurology5th Ed. McGraw‐Hill Inc., New York 1993; 1144–1145
- Warren D. J., Otieno L. S. Carpal tunnel syndrome in patients on intermittent hemodialysis. Post‐Grad. Med. J. 1975; 51: 450–452
- Holtmann B., Anderson C. B. Carpal tunnel syndrome following vascular shunt for hemodialysis. Arch. Surg. 1977; 112: 65–66, [PUBMED], [INFOTRIEVE]
- Gilbert M. S., Robinson A., Baez A., Gupta S., Glabman S., Haimov M. Carpal tunnel syndrome in patients who are receiving long‐term renal hemodialysis. J. Bone Jt. Surg. 1988; 70A: 1145–1153
- Corradi M., Paganelli E., Pavesi G. Carpal tunnel syndrome in long‐term hemodialyzed patients. J. Reconstr. Microsurg. 1989; 5: 103–110, [PUBMED], [INFOTRIEVE]
- Mancusi‐Ungaro A., Corres J. J., Dispaltro F. Median carpal tunnel syndrome following a vascular shunt procedure in the forearm: case report. Plast. Reconstr. Surg. 1976; 57: 96–97
- Reinstien L., Reed W. P., Sadler J. H., Baugher W. H. Peripheral nerve compression by brachial artery‐basilic vein vascular access in long‐term hemodialysis. Arch. Phys. Med. Rehabil. 1984; 65: 142–144, [CSA]
- Wilbourn A. J., Furlan A. J., Hulley W., Ruschhaupt W. Ischemic monomelic neuropathy. Neurology 1983; 33: 447–451, [PUBMED], [INFOTRIEVE]
- Riggs J. E., Moss A. H., Labosky D. A., Liput J. H., Morgan J. J., Gutman L. Upper extremity ischemic monomelic neuropathy: a complication of vascular access procedures in uremic diabetic patient. Neurology 1989; 39: 997–998, [PUBMED], [INFOTRIEVE]
- Hye R. J., Wolf Y. G. Ischemic monomelic neuropathy: an under‐recognized complication of hemodialysis access. Ann. Vasc. Surg. 1994; 8: 578–582, [PUBMED], [INFOTRIEVE], [CSA]
- Redfern A. B., Zimmerman N. B. Neurologic and ischemic complication of upper extremity vascular access for dialysis. J. Hand Surg. 1995; 20: 109–204
- Wytrzes L., Markley H. G., Fisher M., Alfred H. J. Brachial neuropathy after brachial artery antecubital vein shunt for chronic hemodialysis. Neurology 1987; 37: 1398–1400, [PUBMED], [INFOTRIEVE]
- Makkar R. K., Kochar D. K. Somatosensory evoked potentials (SSEPs), sensory nerve conduction velocity (SNCV) and motor nerve conduction velocity (MNCV) in chronic renal failure. Electromyogr. Clin. Neurophysiol. 1994; 34: 295–300, [PUBMED], [INFOTRIEVE], [CSA]
- Bazzi C., Pagani C., Sorgato G., Albonico G., Fellin G., D'Amico G. Uremic polyneuropathy: a clinical and electrophysiological study in 135 short‐ and long term hemodialyzed patients. Clin. Nephrol. 1991; 35: 176–181, [PUBMED], [INFOTRIEVE]
- Bolton C. F., Young G. B. Uremic neuropathy. Neurological Complication of Renal Disease. Butterworth, Boston 1990; 76–107
- Hassan K., Simri W., Rubinchik I., Manelis J., Gross B., Shasha S. M., Kristal B. Effect of erythropoietin therapy on polyneuropathy in predialytic patients. J. Nephrol. 2003; 16(1), (in press)
- Funck‐Brentano J. L., Cueille G. F., Man N. K. A defiance of the middle molecule hypothesis. Kidney Inter., Suppl. 1978; S31–S35
- Babb A. L., Ahmad S., Bergstrom J., Scribner B. H. The middle molecule hypothesis in perspective. Am. J. Kidney Dis. 1981; 1: 46–50, [PUBMED], [INFOTRIEVE]
- Klein C., Halevy A., Gandelman‐Marton R., Halpern Z., Weissgarten J., Averbukh Z., Arlazoroff A. Nerve conduction abnormalities in the arms of patients with arteriovenous fistula. Ren. Fail. 1996; 18(1)85–89, [PUBMED], [INFOTRIEVE], [CSA]