Abstract
Group II A phospholipase A2 (PLA2) produces many inflammatory lipid mediators, and the elevation in the level during sepsis has been correlated positively with the decrease in the arterial blood pressure. We studied the effect of large‐pore continuous venovenous hemodiafiltration (LP‐CVVHDF) on the plasma PLA2 concentration and the clearance mechanism during septic acute renal failure. The subjects were 10 consecutive patients with septic acute renal failure receiving CVVHDF. Simultaneous samples of arterial, and filter inlet and outlet blood, and ultradiafiltrate were collected before starting CVVHDF (0 hr), and 4 hr, 12 hr and 24 hr after starting CVVHDF. PLA2 activity was measured in plasma and ultradiafiltrate. We eluted PLA2 bound to hemofilter from patient and the classification of PLA2 type of eluting solution and ultradiafiltrate was done using Western blot analysis. Plasma clearance (mL/min) was 28.1 ± 7.6 at 4 hr, 23.2 ± 8.9 at 12hr and 17.5 ± 8.0 at 24 hr. Plasma clearance at 4 hr was higher than that at either 12 hr or 24 hr. Plasma clearance mainly consisted of adsorption by LP‐CVVHDF. The changes in arterial plasma PLA2 activity were not statistically significant. One mg/mL of heparin eluted PLA2 bound to the large‐pore hemofilter. The PLA2 in eluting solution and in ultradiafiltrate were identified as an approximately 70 kD band in Western blot analysis using anti‐human secretory II A‐PLA2 monoclonal antibody. The results show that circulating PLA2 can be removed by adsorption with LP‐CVVHDF to some extent and that plasma PLA2 activity is not significantly decreased. Because PLA2 clearance with LP‐CVVHDF is estimated as < 1% of total body PLA2 clearance, LP‐CVVHDF could not be a clinically efficient therapy to remove the circulating PLA2.
Introduction
Group II A phospholipase A2 (PLA2), a 14kD enzyme, is a key enzyme in the production of lipid mediators (prostaglandin I2, thromboxane A2, leukotriene B4, platelet‐activating factor, etc.) from membrane phospholipids when the inflammatory cascade is activated.Citation[1] PLA2 activity was reported to be elevated 35‐fold in the plasma of septic patients, and the measurement of plasma PLA2 is recommended to estimate the individual risk for occurrence of lethal multiple organ failure.Citation[2] The elevation in the level of plasma PLA2 during sepsis has been reported to correlate positively with the decrease in the mean arterial blood pressure.Citation[3] Thus, the removal of circulating PLA2 might have a therapeutic significance in septic patients.
Continuous venovenous hemodiafiltration (CVVHDF) is well suited to the unstable hemodynamic conditions of septic patients complicated with acute renal failure. Although the removal of various inflammatory mediators by CVVHDF became very attractive therapy, it is controversial whether CVVHDF could remove these mediators.Citation[4] The removal of cytokines by CVVHDF is mainly due to hemofilter membrane adsorption, and the adsorption is estimated according to calculation from differences between prefilter and postfilter concentrations of cytokines.Citation[5]
Large‐pore CVVHDF (LP‐CVVHDF) has a larger pore size than the previous standard membrane (cutoff point: 30–50 kD) to permit efficient elimination of various soluble mediators with 10 kD–50 kD molecular sizes, and was reported to improve cardiovascular function during endotoxin shock.Citation[6] However, it is unknown whether LP‐CVVHDF could remove circulating PLA2 clinically effectively.
The present study was carried out to determine if PLA2 could be removed by LP‐CVVHDF clinically effectively, to clarify the clearance mechanisms and to prove directly that the hemofilter membrane adsorbs circulating PLA2 in patients with septic acute renal failure.
Materials and Methods
Patients
This study was approved by the Ethics Committee of the Nagasaki University School of Medicine and conducted in the intensive care unit (ICU) of Nagasaki University Hospital from July 1999 to July 2000. Informed written consent was obtained from each patient or the relatives. The subjects were 10 consecutive patients with septic acute renal failure receiving LP‐CVVHDF. Sepsis was diagnosed according to the criteria of American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference.Citation[7] The diagnosis of acute renal failure was based on the decreased renal function in need of dialysis,Citation[8] as defined by a rise of the serum creatinine > 3 mg/100 mL and a urine output < 20 mL/hr despite volume correction and intensive diuretic therapy. The severity of the disease was assessed using APACHE II score.Citation[9] The Glasgow Coma Scale was excluded from the calculation of the APACHE II score, as some patients were sedated and intubated.
Therapy
All patients received conventional intensive care therapy according to clinical requirements. Vasopressor agents (dopamine, dobutamine, norepinephrine, etc.) were adjusted by the attending physician to maintain an adequate mean arterial blood pressure. Antibiotics indicated by microbiological tests were administered intravenously. All intubated patients were sedated with a continuous infusion of buprenorphine and midazolam. The ventilator setting was adjusted by the attending physician to maintain clinically appropriate gas exchange.
CVVHDF
The femoral vein was cannulated for vascular access with an 11‐Fr, double‐lumen catheter. CVVHDF was performed using a large pore polyacrylonitrile (PAN) hemofilter (APF‐06S, Asahimedical, Tokyo, Japan). This membrane has the cut‐off point of approximately 55–65 kD, the mean pore size of 8.3 nm and the effective surface area of 0.6 m2. The study was carried out during the first 24 hr of treatment with the single hemofilter. Blood was pumped through the hemofilter at 80 mL/min (QB) by a CVVHDF peristaltic pump system, and was then returned to the circulation. We used a bicarbonate dialysate. The bicarbonate dialysate was simultaneously infused through the hemofilter, countercurrent to blood flow, at a constant rate of 500 mL/hr (QD). The ultrafiltration rate was 500 mL/hr (QF) and the same bicarbonate replacement solution was administered at a rate of 400–500 mL/hr (QR) in a postfilter fashion. The extracorporeal circuit was protected against coagulation with nafamostat mesylate (Futhan, Toriiyakuhin, Tokyo, Japan) or heparin in the afferent limb. The infusion of anticoagulants was adjusted to maintain the activated clotting time (ACT) about 150 sec. Simultaneous samples of arterial, and filter inlet and outlet blood, and ultradiafiltrate were collected before starting CVVHDF (0 hr), and 4 hr, 12 hr and 24 hr after starting CVVHDF. At each point, hemodynamic determinations, arterial blood gas analysis and hematological analysis were performed.
Control
We collected arterial samples from 7 control patients (age 56–73, 4 females and 3 males) who underwent total knee replacement surgery without collagen disease. After general anesthesia and cannulation into radial artery, we drew samples for PLA2 assay.
PLA2 Analysis
Samples were centrifuged at 3000 rpm for 10 min, plasma was diluted 50‐fold in buffer consisting of 50 mM Tris, 150 mM NaCl, 1 mM EDTA and 1 mM EGTA, that contained the protease inhibitors, 20 µM leupeptin and 0.1 mM phenylethyl sulfonyl fluoride (PMSF). Diluted plasma and ultradiafiltrate were stored at − 80°C until assayed.
PLA2 activity was measured as the previously described manner with some modification.Citation[10] L‐3‐phosphatidylethanolamine, 1‐acyl‐2‐ [1–14C] arachidonyl (PE) (Amersham, Buckinghamshire, UK) was used as exogenous substrate, which was dried under N2 and resuspended in ethanol. The PLA2 assay buffer (100 µL) contained 75 mM Tris‐HCl, 10 mM CaCl2, and 0.22 nmol of the PE (∼ 25,000 cpm) at pH 9.0. The reaction was carried out at 37°C for 30 min and was stopped by adding 0.56 mL of Dole's reagent: 48.75% isopropyl alcohol, 50% n‐heptane, 1.25% 1N H2SO4 in water. Arachidonic acid (AA) was extracted in the following manner. Water, 0.11 mL, was added and the sample was vortexed and centrifuged at 10,000g for 5 min. A volume of 0.15 mL of the upper phase was transferred to a new tube to which 50 µL silica gel and 0.8 mL of n‐heptane were added. The samples were vortexed and centrifuged again for 5 min each. A volume of 0.8 mL of supernatant was then counted in a liquid scintillation counter. PLA2 activity was expressed as pmol of radiolabeled AA released from PE per min per mL of plasma.
Calculations
The following formulas were used:
Plasma flow (mL/min)
Clearance (mL/min)
Elution of PLA2 Bound to Large‐Pore Hemofilter
One LP‐CVVHDF hemofilter was removed from No.9 patient 4 hr after starting CVVHDF the next day after time‐course experiment. One thousand mL of acetate Ringer solution was pumped through the hemofilter at 100 mL/min without convection or dialysis, to wash the hemofilter. Ten 5‐mL fractions every 100 mL of washed solution (fraction number: F1 to F10) were collected. And then, two hundred mL of saline including 1 mg/mL heparinCitation[11] was pumped through the hemofilter at 100 mL/min to elute PLA2 from the hemofilter. Two 5‐mL fractions every 100 mL of eluting solution (fraction number: H1, H2) were collected. The protease inhibitors, 20 µM leupeptin and 0.1 mM PMSF, were added to each fraction and fractions were centrifuged at 3000 rpm for 10 min. Protein concentration of each fraction was measured using a protein analysis kit (Bio‐Rad Laboratories, Richmond, CA) with bovine serum albumin as a standard and PLA2 activity of each fraction was assayed.
Western Blot Analysis
Two mL of ultradiafiltrate from No. 9 patient was concentrated to 200 µL using Centricon 10 (Amicon, Danvers, MA). Proteins in F10, H1 and concentrated ultradiafiltrate were denatured and subjected to sodium dodecyl sulfate (SDS)‐polyacrylamide gel electrophoresis (PAGE) (15% polyacrylamide gradient gel).Citation[12] Human synovial sPLA2 (purified group II A PLA2) (Cayman Chemical, Ann Arbor, MI) was used as a standard. After SDS‐PAGE, separated proteins were electrophoretically transferred to a polyvinylidene fluoride (PVDF) (PVDF‐PLUS, Micron Separations, Westborough, MA) membrane in 25 mM Tris‐HCl (pH = 8.3), 190 mM glycine and 20% methanol. Nonspecific binding of anti‐human group II A PLA2 antibody to PVDF membrane was prevented by preincubation of the membrane in 5% skim milk in Tris‐buffered saline (TBS; 25 mM Tris‐HCl, pH 8.0, 137 mM NaCl) for an hr at room temperature. The blocked PVDF membrane was incubated with a 1: 200 dilution of a mouse anti‐human group II A PLA2 antibody (Upstate Biotechnology, Lake Placid, NY) for 2 hr at room temperature with constant shaking. Unbound antibodies were removed with three washes of TBST (TBS containing 0.5% Tween 20). The PVDF membrane was incubated with a 1:3000 dilution of a rabbit anti‐mouse immunoglobulin, peroxidase‐linked species‐specific whole antibody (Wako, Osaka, Japan) for 1 hr at room temperature. The sites of antibody binding were developed with an enhanced chemiluminescence system (LuminGLO, New England Biolabs, Beverly, MA).
Statistical Analysis
All data of PLA2 activity were obtained as the average of triplicate measurements. Results were presented as mean ± SD. Results were evaluated by Kruskal‐Wallis and Wilcoxon test with P < 0.05 regarded as significant.
Results
Patients
The characteristics of the patients are summarized in . The mean APACHE II score, mean age (years) and mean body weight (kg) were 16.5 ± 3.9, 70.8 ± 9.6 and 50.7 ± 10.6, respectively. Four patients (40%) survived to leave the ICU. Six patients (60%) died from irreversible multiple organ distress syndrome (MODS) following sepsis.
Table 1. Patient Characteristics
Control
In the control group, the plasma PLA2 activity was 2.5 ± 1.9 pmol/min/mL.
Hemofilter Data
The kinetic property of PLA2 under LP‐CVVHDF is shown in . There were no significant differences in the plasma PLA2 activity among the time point. There were no significant changes in PLA2 sieving coefficient of LP‐CVVHDF during the time course. Plasma PLA2 clearance (mL/min) by LP‐CVVHDF was 28.1 ± 7.6 at 4 hr, 23.2 ± 8.9 at 12 hr and 17.5 ± 8.0 at 24 hr. Plasma PLA2 clearance at 4 hr was higher than that at either 12 hr or 24 hr. Ultradiafiltrate PLA2 clearance by LP‐CVVHDF did not change throughout the time course. Adsorption PLA2 clearance (mL/min) at 4 hr (27.7 ± 7.9) was higher than that at either 12 hr (22.3 ± 8.8) or 24 hr (16.0 ± 8.3).
Table 2. Parameters of Phospholipase A2 Clearance (mean ± SD) of LP‐CVVHDF
Elution of PLA2 Bound to Large‐Pore Hemofilter
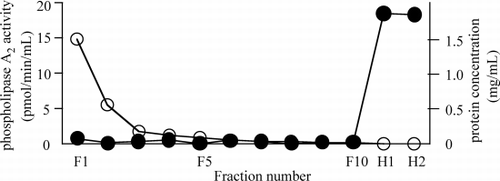
As shown in , the washing solution and eluting solution diluted plasma protein concentration in each fraction, gradually. Although PLA2 activities in the washed solution fractions except F1 were close to 0, PLA2 activities in the eluted solution fractions elevated to above 15 pmol/min/mL. Thus, 1 mg/mL of heparin in eluting solution would have eluted PLA2 bound to large pore hemofilter.
Figure 1. Phospholipase A2 (PLA2) activity and protein concentration after heparin elution from large‐pore hemofilter. One hemofilter was washed with 1 L of acetate Ringer solution and ten 5‐mL fractions every 100 mL of washed solution (fraction number: F1 to F10) were collected. PLA2 bound to hemofilter was eluted with 200 mL of saline including 1 mg/mL heparin and two 5 mL‐fractions every 100 mL of eluting solution (fraction number: H1, H2) were collected. Protein concentration (open circle) and PLA2 activity (solid circle) of each fraction were assayed.
Western Blot Analysis
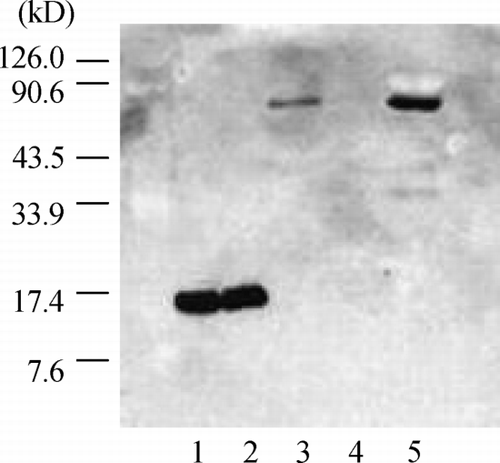
In order to confirm that eluted PLA2 from the hemofilter was group II A PLA2, the classification of PLA2 type was done using Western blot analysis with anti‐human group II A PLA2 monoclonal antibody. As shown in , the purified II A PLA2 and purified PLA2 including 1mg/mL heparin were identified as an approximately 14 kD band in Western blot analysis, and the PLA2 in eluting solution (H‐1) was identified as an approximately 70 kD band. There was no band in F‐10. The PLA2 in the ultradiafiltrate was identified as an approximately 70 kD.
Figure 2. Western blot analysis of the eluted phospholipase A2 (PLA2) using anti‐human group IIA PLA2 monoclonal antibody. The purified IIA PLA2 (lane 1) and purified PLA2 including 1 mg/mL heparin (lane 2) were identified as one approximately 14 kD band, and the PLA2 in eluting solution (H‐1) (lane 3) was identified as an approximately 70 kD band. There was no band in washing solution (F‐10) (lane 4). The PLA2 in the ultradiafiltrate was identified as an approximately 70 kD band (lane 5).
Discussion
The present results show that plasma PLA2 activity can be removed by adsorption with LP‐CVVHDF to some extent and the efficiency of adsorption depends on the time, and that plasma PLA2 activity is not significantly decreased. Because PLA2 sieving coefficient of LP‐hemofilter is less than 0.1, the hemofilter cannot convey circulating PLA2.
Although there was no previous report concerning kinetic properties of group II A PLA2 in humans, kinetic properties of the enzyme in rat were reported previously.Citation[13] Murakami et al. injected purified group II A PLA2 labeled with 125I into the rat intravenously, and measured the half‐life of the enzyme. They demonstrated that the radioactivity disappeared rapidly from the blood stream, and that it remained 17.4% of the injected radioactivity 1 min and 0.4% 10 min after injection. Assuming that the body weight of rat, total blood volume and hematocrit are 0.45kg, 7% and 47%, respectively, total body PLA2 clearance is calculated using one‐compartment model. Total body PLA2 clearance in the rat was 26.5 mL/min. Adapting the clearance in the rat to human, total body PLA2 clearance in human corrected by body weight is about 3000 mL/min. PLA2 clearance with LP‐CVVHDF is estimated as < 1% of total body PLA2 clearance in human. With regard to drug removal, it is generally accepted that extracorporeal clearance is clinically significant if its contribution to total body clearance exceeds 25–30%.Citation[4]
Yekebas et al. induced pancreatitis by a combined intraductal injection of sodium taurocholate and enterokinase in pig.Citation[14] They compared the pigs treated with continuous venovenous hemofiltration (CVVHF) with those without CVVHF. CVVHF significantly prolonged survival time and improved hemodynamic parameters and gas exchange. CVVHF significantly attenuated an increase in PLA2 concentrations induced by pancreatitis. The sieving coefficient of their membrane was 0.7–0.8. Estimating from the sieving coefficient of myoglobin (17.8kD)Citation[15] which is a larger molecular weight than II A PLA2 (14 kD), the expected II A PLA2 sieving coefficient of LP‐CVVHDF is greater than 0.85. However, the present PLA2 sieving coefficient of LP‐hemofilter is less than 0.1. Our present result shows that purified group II A PLA2 has a molecular weight of 14 kD. However, the circulating PLA2 filtered to the ultradiafiltrate and bound to the membrane whose molecular weight are approximately 70 kD precludes important passage through the LP‐hemofilter.
It has been reported that the activity of group II A PLA2 increases in the serum of septic patients. Murakami et al. demonstrated that group II A PLA2 had capacities for binding to cell surface and heparin Sepharose chromatography, and that high concentrations of either salt (1M) or heparin (1 mg/mL) solubilized membrane associated group II A PLA2.Citation[11] We solubilized circulating PLA2 bound to hemofilter and Western blot analysis shows that solubilized PLA2 is identified as an approximately 70 kD band. PLA2 in the ultradiafiltrate is identified as an approximately 70 kD band. This monoclonal antibody cannot cross‐react with other mammalian PLA2, and Western blot analysis shows that purified PLA2 including 1 mg/mL heparin were identified as one approximately 14 kD band. Circulating group II A PLA2 might be bound to some substance or be an oligomer, according to the present Western blot results.
In conclusion, circulating group II A PLA2 can be removed by adsorption with LP‐CVVHDF to some extent and the efficiency of adsorption depends on the time. However, LP‐CVVHDF cannot affect plasma PLA2 activity, and thus LP‐CVVHDF is not a clinically efficient therapy to remove the circulating PLA2.
References
- Bulger E. M., Maier R. V. Lipid mediators in the pathophysiology of critical illness. Crit. Care Med. 2000; 28(4)N27–N36, Suppl.[PUBMED], [INFOTRIEVE], [CSA]
- Uhl W., Beger H. G., Hoffmann G., Hanisch E., Schild A., Waydhas Ch., Entholzner E., Müller K., Kellermann W., Vogeser M., Leskopf W., Zügel M., Busch E. W., Büchler M. W. A multicenter study of phospholipase A2 in patients in intensive care unit. J. Am. Coll. Surg. 1995; 180(3)323–331, [PUBMED], [INFOTRIEVE], [CSA]
- Vadas P., Pruzanski W., Stefanski E., Sternby B., Mustard R., Bohnen J., Fraser I., Farewell V., Bombardier C. Pathogenesis of hypotension in septic shock: correlation of circulating phospholipase A2 levels with circulatory collapse. Crit. Care Med. 1988; 16(4)1–7, [PUBMED], [INFOTRIEVE]
- Schetz M., Ferdinande P., Van den Berghe G., Verwaest C., Lauwers P. Removal of pro‐inflammatory cytokines with renal replacement therapy: sense or nonsense?. Intensive Care Med. 1995; 21(2)169–176, [PUBMED], [INFOTRIEVE], [CSA]
- De Vriese A. S., Colardyn F. A., Philippe J. J., Vanholder R. C., DeSutter J. H., Lameire N. H. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol. 1999; 10(4)846–853, [PUBMED], [INFOTRIEVE], [CSA]
- Kline J. A., Gordon B. E., Williams C., Blumenthal S., Watts J. A., Diaz‐Buxo J. Large‐pore hemodialysis in acute endotoxin shock. Crit. Care Med. 1999; 27(3)588–596, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- American College of Chest Physicians/ Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992; 20(6)864–874
- Thadhani R., Pascual M., Bonventre J. V. Acute renal failure. N. Engl. J. Med. 1996; 334(22)1448–1460, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. APACHE II: a severity of disease classification system. Crit. Care Med. 1985; 13(10)818–829, [PUBMED], [INFOTRIEVE]
- Nakamura H., Kim D. K., Philbin D. M., Peterson M. B., Debros F., Koski G., Bonventre J. V. Heparin‐enhanced plasma phospholipase A2 activity and prostacyclin synthesis in patients undergoing cardiac surgery. J. Clin. Invest. 1995; 95(3)1062–1070, [PUBMED], [INFOTRIEVE], [CSA]
- Murakami M., Shimbara S., Kambe T., Kuwata H., Winstead M. V., Tischfield J. A., Kudo I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. J. Biol. Chem. 1998; 273(23)14411–14423, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Song C. H., Choi J. S., Kim D. K., Kim J. C. Enhanced secretory group II PLA2 activity in the tears of chronic blepharitis patients. Invest. Ophthalmol. Vis. Sci. 1999; 40(11)2744–2748, [PUBMED], [INFOTRIEVE], [CSA]
- Murakami M., Kudo I., Inoue K. In vivo release and clearance of rat platelet phospholipase A2. Biochim. Biophys. Acta 1989; 1005(3)270–276, [PUBMED], [INFOTRIEVE]
- Yekebas E. F., Treede H., Knoefel W. T., Bloechle C., Fink E., Izbicki J. R. Influence of zero‐balanced hemofiltration on the course of severe experimental pancreatitis in pigs. Ann. Surg. 1999; 229(4)514–522, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Katayama H. Comparison of continuous renal replacement therapy by clearance measurement. Journal of the Japanese Society of Intensive Care Medicine 1998; 5(2)115–121
