Abstract
The incidence of renal calculi has been evaluated to be 25% in urogenital tuberculosis patients. The stone could be caused due to the host, the pathogenic organism, or possibly by the treatment. Studies were carried out to find out the efficacy of vitamin E supplementation in reducing the risk of stone formation in renal tuberculosis patients. The study constituted four groups, Group I with 30 normal volunteers, the second group comprised of 36 renal tuberculosis patients (GuTb) a day before treatment. Third group comprised of 24 patients with regular anti tuberculosis drug regimen for sixty days. In the fourth group, 12 patients were treated with anti tuberculosis drug regimen along with supplementation of antioxidant vitamin E (200 mg/day) for sixty days. Hyperuricosuria and hypercalciuria were observed in group II and group III patients, along with increased excretion of oxalate and creatinine, accompanied by decreased excretion of inhibitors such as citrate and glycosaminoglycans (GAGs). Renal damage was evident with increased leakage of Lactate dehydrogenase (LDH), Alkaline phosphatase (ALP) and γ‐Glutamyl transferase (γ‐GT) in renal tuberculosis patients. From the results of the above study, it is obvious that increased urinary oxalate levels leads to cellular damage in GuTb patients, which is a prerequisite for crystal retention as revealed by the elevated urinary marker enzymes. Antioxidant therapy prevents membrane injury thereby reducing the risk of stone formation. Hence vitamin E supplementation has a salubrious effect in preventing stone forming tendency with routine anti tuberculosis drug regimen.
Introduction
The worldwide prevalence of tuberculosis is still high and has remained almost unchanged over the past century. Twenty percent of patients with tuberculosis develop an extra pulmonary manifestation over time, the most common site being the genitourinary tract.Citation[1] Pathogenesis begins with the initial localization of the tubercula bacilli in the cortical glomeruli causing mechanical stress, which leads to alteration in cell morphology.Citation[2] In these renal tuberculosis patients a high incidence of calculi of up to 24.3% has been reported.Citation[3], Citation[4] The exact pathogenesis of stone formation is not known. However, it is understood that calculi would be either due to secondary infection or with a specific chemotherapy.Citation[5] The most common type of stone is composed of calcium, either as oxalate or phosphate salt alone or as a combination of both.Citation[3] Apart from calcium and oxalate supersaturation, membrane injury is considered to be the prime candidate for binding of crystals.Citation[6] Sandhya et al.,Citation[7] have demonstrated that the excess urinary nephrotoxic challenge by aminoglycosides increases the risk of stone formation in experimental hyperoxaluric rats.Citation[8] Similarly, there are evidences to suggest that drug induced toxicity in renal tuberculosis patients leads to cell injury.Citation[2] Studies have proved that supplementation of vitamin E can protect the membrane from injury thereby preventing stone formation in experimental urolithiasis.Citation[9] The role of vitamin E in urogenital tuberculosis associated urolithiasis with their relationship on the excretion of substances considered to be risk factors for stone formation has not been well characterized. Hence the present study was undertaken to determine the efficacy of vitamin E in preventing membrane damage originated either endogenously or exogenously and to evaluate the associated risk factors of urolithiasis.
Materials and Methods
Blood and urine samples were collected from patients suffering from urogenital tuberculosis, admitted to the Department of Urology, Stanley Medical College and Hospital, Chennai. All the chemicals used were of analytical grade.
Thirty‐six patients (26 male and 10 female with the mean age of 40.1 ± 9.09) and age matched normal subjects were included in the study. Group I—normal subjects (n = 30, 20 male and 10 female); Group II—GuTb patients a day before treatment (n = 36, 26 male and 10 female); Group III—GuTb patients after treatment with Isoniazid 300 mg, Rifampicin 450 mg and Pyrazinamide 1.5 g per day for 60 days (n = 24, 18 male and 6 female) and Group IV patients—GuTb patients supplemented with vitamin E 200 mg/day along with regular chemotherapy (n = 12, 8 male and 4 female) for 60 days.
The heparinized blood was collected, centrifuged and the plasma was used for the estimation of vitamin E by Barker and Frank.Citation[10]
Twenty‐four hour urine sample was collected in polyethylene bottles in a ice cold condition for the assay of stone risk profiles such as calcium,Citation[11] oxalate,Citation[12] uric acid,Citation[13] phosphorus,Citation[14] citrateCitation[15] and GAGs.Citation[16] Excretion of urinary enzymes LDH,Citation[17] ALPCitation[18] and γ‐GTCitation[19] were monitored.
Statistical analysis was carried out using statistical package for social science version 7.5 and the data are expressed as mean ± SD with significance compared at 0.05 level.
Results
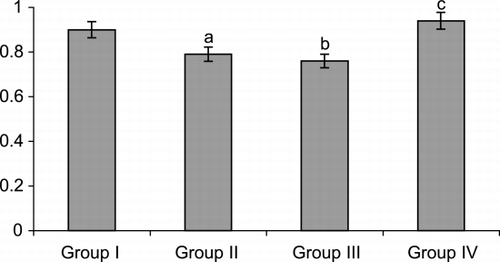
The plasma vitamin E was found to be significantly lower in untreated and treated GuTb patients. Approximately 15% decrease in the levels were observed in both the groups. Vitamin E supplementation brings back the levels to normal ().
Figure 1. Level of vitamin E in urogenital tuberculosis patients and on treatment with anti Tb drugs.
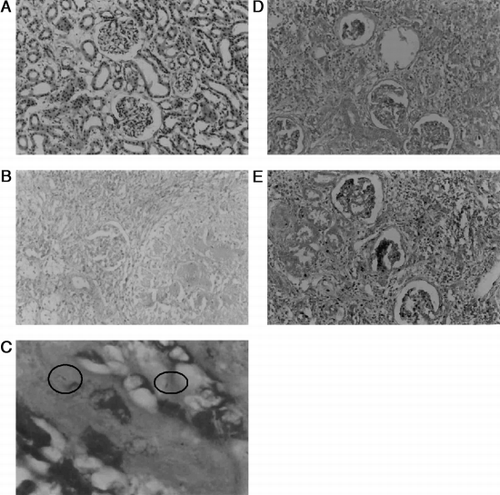
Biopsy sections of kidney in Group II patients show granulomatous inflammation, giant cells with caseation and PAS staining revealed the presence of mycobacterium. Renal tuberculosis was controlled on treatment with antituberculosis drug, however tubular impairment and morphological changes were still noticed on chemotherapy in Group III patients. Cellular morphology was found to be normal in the patients supplemented with vitamin E along with anti Tb drugs ().
Figure 2. A. Histology section of kidney normal architecture. B. Kidney section showing granulomatous inflammation, giant cells and caseation in GuTb patients. C. Presence of Mycobacterium tuberculosis (PAS staining). D. Histological section showing tubular impairment and abnormal granuloma in GuTb patients treated with antituberculosis drugs. E. Kidney section of GuTb patients treated along with vitamin E.
The urinary enzyme activities of ALP, LDH and γ‐GT were found to be 109–130% of normal in Group II patients and still increased by about 198–262% in Group III patients (). Enzyme activities were found to be well within normal limits, when antioxidant vitamin E was supplemented along with their regular drug regime.
Table 1. Urinary Excretion of Renal Marker Enzymes
depicts the change in excretion pattern of stone forming constituents such as calcium, oxalate, uric acid, protein, creatinine, GAGs and citrate in GuTb patients before and after chemotherapy along with supplementation of antioxidant vitamin E. Excretion of stone promoters such as calcium, oxalate and uric acid were found to be increased in GuTb patients and they were further elevated on chemotherapy. Similarly, decreased levels of inhibitors such as GAGs and citrate were noticed. These risk factors were normalized in Group IV patients on treatment for renal tuberculosis with regular chemotherapy supplemented with vitamin E.
Table 2. Stone Risk Profile Determined in Urine of Urogenital Tuberculosis Patients Before and After Treatment of Regular Anti Tb Drugs Along with Vitamin E
Discussion
Urinary chemistry is one of the important factors in determining the supersaturation and molecules responsible for the stone genesis.Citation[20] Hence, the study of urinary chemistry in renal tuberculosis patients will be useful in assessing the risk of stone formation.
Urinary oxalate an important determinant of stone formationCitation[21] was found to be raised in GuTb patients and further increased on treatment with anti Tb drugs. Increased urinary oxalate levels have been associated with oxidative stressCitation[22] and this might be due to the increased conversion of oxalate precursors to oxalate by LDH and XO, which are elevated under nephrotoxic conditions.Citation[23] Further, in group III patients, exogenous oxalate might also be increased due to hyper absorption of oxalate via the brush border membrane of intestine, which is found to be damaged by antituberculosis drugs.Citation[5]
Several hypotheses have been advocated that cellular injury is one of the predisposing factors for crystal retention,Citation[6] which is obvious, by the increased excretion of marker enzymes in the urine.Citation[24] Most of the urinary enzymes originating in the kidneys are localized to specific portions and cellular components of the nephron.Citation[25] Tubular injury is evident in GuTb as well as in patients treated with anti Tb drugs alone by the increased excretion of renal tubular cytoplasmic enzyme, LDH. Further, the patients were found to have increased activities of brush border membrane enzymes ALP and γ‐GT which is analogous to the observation made by Malini et al.,Citation[26] in urolithic rats. Oxalate is known to induce lipid peroxidation,Citation[8] the increased urinary oxalate levels in group II and group III patients might be responsible for increased membrane damage. Antituberculosis drugs like rifampicin, isoniazid and pyrazinamide are also nephrotoxic in nature.Citation[27] This still aggravates the lipid peroxidation leading to increased membrane damage in group III patients and thereby membrane leak. The damaged membrane facilitates the entry of calcium into the cell from extra cellular fluids and in condition like free radicals accumulation, cellular and mitochondrial calcium in the kidney will be raised.Citation[28] Increased calcium and oxalate levels within the tissue increases the supersaturation with respect to calcium oxalate crystallization, thereby favoring the formation of crystals within the cell. Further, membrane damage is shown to release cell debris. Cell debris and nucleated crystals can be retained in the damaged urothelium,Citation[29] which can grow aggregate to a mature stone.
Hyperuricosuria observed in group III patients may also be due to the intake of drug pyrazinamide.Citation[30] Increased levels of uric acid moreover interfere with calcium oxalate solubilityCitation[31] and reduce the inhibitory activity of glycosaminoglycans.Citation[32] Further, urinary pH was also found to be acidic in Group II and III patients which is recognized not only in the pathogenesis of uric acid stone formationCitation[32] but also in hypocitraturia.Citation[33] The decrease in citrate is frequently encountered in patients with nephrolithiasis and citrate therapy advocated to stone patients substantiates its role in stone formation.Citation[34]
Several investigations have shown that membrane damage can be taken care by supplementation of antioxidants in various pathological conditions.Citation[35] Antioxidants like vitamin E, glutathione monoester or methionine or lipoic acid normalized the cellular antioxidant system, prevented precipitation of salts in the rat kidneyCitation[6] and reduced oxalate excretion in stone patients.Citation[36] Similarly, the present study has also proved that supplementation of vitamin E protects the membrane and prevents cellular dysfunction such that reducing the risk of stone formation in renal tuberculosis.
References
- Lenk S., Schroeder J. Genitourinary tuberculosis. Curr. Opin. Urol. 2001; 11: 93–96, [CSA]
- Chijioke A. Current concepts on pathogenesis of renal tuberculosis. West Afr. J. Med. 2001; 20(2)107–110
- Lenk S., Schubert G., Oesterwitz H., Brien G. Urolithiasis associated with urogenital tuberculosis clinical and mineralogical aspects. Urol. Res. 1988; 16(3)157–159, [CROSSREF]
- Dolev E., Bass A., Nussinowitz N. Frequent occurrence of renal calculi in tuberculosis kidney in Israel. Urology 1985; XXVI(6)544–545, [CROSSREF]
- Yadav T. P., Gupta V. K., Khanna R., Autar K., Mishra S. Calcification in renal tuberculosis. Indian Pediatr. 1995; 32: 581–585, [CSA]
- Selvam R. Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol. Res. 2002; 30(1)35–47, [CSA], [CROSSREF]
- Sandhya P., Mohandass S., Varalakshmi P. Role of DL α‐lipoic acid in gentamicin induced nephrotoxicity. Mol. Cell Biochem. 1995; 145: 11–17, [CSA]
- Hackett R. L., Shevock P. N., Khan S. R. Cell injury associated calcium oxalate crystalluria. J. Urol. 1990; 144: 1535–1536
- Selvam R., Ravichandran V. Restoration of tissue antioxidants and prevention of renal stone deposition in vitamin B6 deficient rats fed with vitamin E or Methionine. Ind. J. Exp. Biol. 1993; 31: 882–887, [CSA]
- Barker H., Frank O. Clinical Vitaminology Methods and Interpretation. Interscience Publishers John Wiley and Sons, Inc., New York 1968; 172–174
- Mustafa M. A., Medeirous D. M. Proximate composition mineral content and fatty acids of cat fish (Ictalurus punctatus ratinesque) for different seasons and cooking methods. J. Food Sci. 1985; 50: 585–588
- Hodgkinson A., Williams A. An improved colorimetric procedure for urine oxalate. Clin. Chim. Acta 1972; 36: 127–132, [CROSSREF]
- Caraway W. T. Uric acid. Standard Methods of Clinical Chemistry, D. Seligson. Academic Press, New York 1963; Vol. 4: 239–247
- Fiske C. H., Subbarow Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925; 66: 375–381
- Rajagopal A. A simple colorimetric estimation of citric acid in urine. J. Indian Exp. Biol. 1984; 22: 391–392
- Dembure P. P., Roesel R. A. Screening for mucopolysaccharidoses by analysis of urinary glycosaminoglycans. Techniques in Diagnostics Human Biochemical Genetics: A Laboratory Manual, F. A. Hummes. Wiley Liss, Inc., New York 1991; 77–86
- King J. Lactate dehydrogenase. Practical Clinical Enzymology, Doran. Nostrand Company Limited, London 1965; 83–93
- King J. The hydrolases acid and alkaline phosphatase. Practical Clinical Enzymology, D. Van. Nostrand company limited, London 1965; 191–208
- Orlowshi S., Meister A. Isolation of γ‐glutamyl transpeptidases from dog kidney. J. Biol. Chem. 1965; 20: 338–347
- Sakhaee K., Huet B. A., Moe O. W., Pak C. Y.C. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kid. Int. 2002; 62: 971–979, [CROSSREF]
- Tiselius H. G. Metabolic evaluation of patients with stone disease. Urol. Int. 1997; 59: 131–141, [CSA]
- Scheid C. R., Koul H., Kennington L., Hill W. A., Luber‐Narod J., Jonassen J., Honeyman T., Menon M. Oxalate induced damage to renal tubular cells. Scanning Microsc. 1995; 9: 1097–1107
- Selvam R., Adhirai M. Vitamin E pretreatment prevents cyclosporin A induced crystal deposition in hyperoxaluric rats. Nephron 1997; 75: 77–81, [CSA]
- Muthukumar A., Selvam R. Renal injury medicated calcium oxalate nephrolithiasis: role of lipid peroxidation. Ren. fail. 1997; 19(3)401–408, [CSA]
- Guder W. G., Ross B. D. Enzyme distribution along the nephron. Kidney Int. 1984; 26: 101–111
- Malini M. M., Baskar R., Varalakshmi P. Effect of lupeol, a pentacyclic triterpene, on urinary enzymes in hyperoxaluric rats. Jpn. J. Med. Sci. 1995; 48: 211–220, [CSA]
- ATS‐CDC. Treatment of tuberculosis and tuberculosis infection in adults and children. Am. J. Resp. Crit. Care Med. 1994; 149: 1359–1374, [CSA]
- Schieppati A., Wilson P. D., Burke T. J., Schrieer R. W. Effect of renal ischemia on cortical microsomal calcium accumulation. Am. J. Physiol. 1985; 249: C476
- Khan S. R., Cockrell C. A., Finlayson B. Crystal retention by injured urothelium of the rat urinary bladder. J. Urol. 1984; 132: 153–156
- Inoue T., Ikeda N., Kurasawa T., Sato A., Nakatani K., Ikeda T., Yoshimatsu H. Hyperuricemia and arthralgia during pyrazinamide treatment. Nihon Kokyuki Gakkai Zasshi 1999; 37(2)115–118, [CSA]
- Grover P. K., Ryall R. L., Marshall V. R. Dissolved urate promotes calcium oxalate crystallization: epitaxy is not the cause. Clin. Sci. 1993; 85: 303–307
- Shekarriz B., Stoller M. L. Uric acid nephrolithiasis: current concepts and controversies. J. Urol. 2002; 168: 1307–1314, [CSA], [CROSSREF]
- Pak C. Y.C. Citrate and renal calculi: an update. Miner. Electrolyte Metab. 1994; 20: 371–377, [CSA]
- Selvam R., Bijikurien T. Effect of citrate feeding on free radical induced changes in experimental urolithiasis. Ind. J. Exp.Biol. 1992; 30: 705–710, [CSA]
- Buoncristiani U., Galli F., Rovidati S., Albertini M. C., Campus Canestrari F. Hemodialysis using a vitamin E modified dialysis membrane. Nephron 1997; 77: 57–61, [CSA]
- Anbazhagan M., Hariprasad C., Samudram P., Latha E., Latha M., Selvam R. Effect of oral supplementation of vitamin E on urinary risk factors in patients with hyperoxaluria. J. Clin. Biochem. Nutr. 1999; 27: 37–47
