Abstract
Objective: To compare the effect of lactate vs. bicarbonate‐buffered replacement fluids on electrolyte mass balance during isovolemic continuous veno‐venous hemofiltration (CVVH). Design: Randomized controlled study with double cross over. Setting: Intensive care unit of a tertiary university hospital. Patients and participants: Eight patients with acute renal failure (ARF). Interventions: Isovolemic CVVH (2L/hr of replacement fluid) was performed in random order with either bicarbonate or lactate‐buffered replacement fluid delivered pre‐filter. Measurements and Results: Sodium, potassium, chloride, magnesium, and phosphate, were measured in each sample. There was a mass gain of sodium, which was similar under both conditions (bicarbonate: 23.3 ± 4.9 mmol/hr, lactate: 22.7 ± 3.5 mmol/hr). Mass chloride gains occurred with bicarbonate‐buffered replacement fluid only (12.8 ± 5.3 mmol/hr), while there was an overall net loss of chloride with lactate fluids (− 2.5 ± 5.2 mmol/hr), resulting in a significant difference in chloride mass balance (p < 0.0001). Magnesium mass balance was negative with bicarbonate buffer only (− 0.6 ± 0.2 mmol/hr) and also differed significantly from that obtained with lactate fluids (− 0.1 ± 0.2 mmol/hr, p < 0.0001). Phosphate losses (bicarbonate: − 1.7 ± 0.7 mmol/hr, lactate: − 1.7 ± 0.5 mmol/hr) were equivalent with both buffers. Potassium mass balance was neutral. Conclusions: Mass balance during isovolemic CVVH is significantly affected by the type of replacement fluid administered pre‐filter. Isovolemic CVVH is not isonatremic and the use of bicarbonate‐buffered fluid results in a significant accumulation of chloride and a loss of magnesium.
Introduction
Hemofiltration appears to have a significant and variable impact on the concentration of several electrolytes and on acid–base balance.Citation[1], Citation[2], Citation[3], Citation[4], Citation[5], Citation[6] Some of this effect is likely to depend on the composition of the replacement fluid. In Europe and Australia, the dominant commercially available replacement fluids are either lactate‐buffered or bicarbonate‐buffered. These fluids are not only likely to have a different effect on acid–base physiology; they are also likely to have a different effect on several electrolytes because of their different composition. Such differences might affect electrolyte mass balance even when renal replacement therapy is isovolemic (replacement fluid = ultrafiltration). Thus, it is possible that, with one type of replacement fluid, the accumulation or loss of a given electrolyte might 1) be substantial and 2) differ significantly from that obtained with another fluid. Appreciation of such differences might be important for clinicians faced with unexplained changes in serum electrolyte concentrations over time during CRRT and might help them avoid unnecessary investigations or interventions. Accordingly, we conducted a randomized controlled double‐cross over study to specifically evaluate, compare and quantify these effects with 2 commercially available solutions.
Materials and Methods
Institutional approval was granted by the hospital Ethics Committee. Informed consent was obtained from each patient's next of kin.
Study Design
Eight consecutive patients with established acute renal failure and fulfilling standard criteria for the multi‐organ dysfunction syndromeCitation[7] were studied. Acute renal failure was defined as acute anuria for > 24 hours in the presence of a plasma urea level > 25 mmol/L and of a serum creatinine > 250 µmol/L.
All patients had to have received CVVH for at least 24 hours prior to enrollment. The study patients were randomized to receive either a 2‐hour period of lactate‐buffered CVVH (treatment A) or bicarbonate‐buffered CVVH (treatment B). The order in which (A) or (B) was applied was random and concealed (random number table and sealed opaque envelopes). The first 2‐hour session was followed by cross over to the other treatment. On the following day, the same random order cross over was performed with the first treatment determined in the same way. Thus the treatment schedule on the study days for each patient was at random one of the following: ABAB, BABA, ABBA, BAAB. Prior to the beginning of each 2‐hour treatment session, arterial blood was obtained for baseline measurements. During each session, arterial blood and UF were obtained at one and two hours of operation. The replacement fluid was then changed and the cycle repeated.
Technique of CVVH
Isovolemic CVVH was performed. Vascular access was obtained with 13.5 FG dual lumen catheters (Niagara™, Bard, Ontario, Canada). A BMM 10‐1 machine or a Prisma machine (Gambro, Lund, Sweden) were used with a 1.2m2 AN69 polyacrylonitrile filter (Filtral 12, Hospal, Lyon, France). Blood flow was set at 180–200 ml/min, and ultrafiltrate flow was set at 2 l/h. Lactate‐buffered replacement fluid (Hemofiltration Replacement Fluid, Baxter Healthcare, Sydney, Australia) was delivered pre‐filter at a rate controlled by either an independent volumetric pump (Gemini PC‐2, IMED Corporation, San Diego, CA) or by the volumetric pump of the Prisma machine. Potassium chloride (13.4 mmol) was added to each 5‐liter bag of replacement fluid (final concentration of 3.7 mmol/L) to prevent hypokalemia. A bicarbonate buffered replacement solution was administered during treatment B (Haemosol B, Gambro Australia, Melbourne, Australia). Potassium chloride (18.5 mmol) was added to this fluid to prevent hypokalemia and achieve the same concentration of potassium as in the lactate‐based fluid.
All replacement fluids were warmed for both techniques. For both techniques, circuit anticoagulation was administered according to a previously described protocol.Citation[8]
All fluid therapy not related to hemofiltration was controlled by the attending physician and remained unchanged during the brief study period. Nutritional fluids and medications were also continued unchanged during each treatment.
Measurements
Samples were drawn into plain tubes and sodium, potassium, chloride, total magnesium, and phosphate; concentrations were measured (Hitachi 747 Analyser, Hitachi). Hematocrit and albumin concentrations were also measured on the morning of the study (Hitachi 747 Analyser, Hitachi and Sysmex SE 9000 Analyser, Roche, respectively).
Calculations
Mass balance was simply calculated as the difference between the amount of a given electrolyte administered (concentration in the replacement fluid × amount of fluid given over unit of time) and the amount lost in the ultrafiltrate (concentration in the ultrafiltrate × amount of ultrafiltrate produced over a unit of time).
Statistical Analysis
A commercially available statistical package was used for data analysis (Staview, Abacus Inc, Berkeley, CA). Data are presented as means ± standard deviation. The changes in sieving coefficient and mass transfer with different buffers were compared using Wilcoxon signed rank test. A p value < 0.05 was considered significant.
Results
Eight patients were studied. Patient demographics and baseline serum solute concentrations are presented in . The electrolyte concentrations in the replacement fluids and serum are presented in and respectively.
Table 1. Demographics of Study Patients
Table 2. Solute Concentrations in Bicarbonate and Lactate Buffers (mmol/L)
Table 3. Electrolyte Concentrations in Blood Pre‐ and Post‐CVVH (mmol/L)
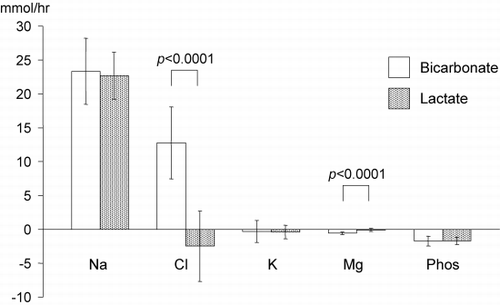
Mass transfer calculations showed significant mass gains or losses of some electrolytes despite isovolemic therapy. The mass transfer values for the various electrolytes are shown in . They demonstrate a significant gain of sodium with both types of replacement fluid (bicarbonate: 23.3 ± 4.9 mmol/hr, lactate: 22.7 ± 3.5 mmol/hr) but a significant gain in chloride with the bicarbonate buffered replacement fluid only (12.8 ± 5.3 mmol/hr), leading to a significant difference in chloride mass balance compared to lactate buffered fluid (− 2.5 ± 5.2 mmol/hr, p < 0.0001). The same bicarbonate fluid also resulted in a significant loss of magnesium of − 0.6 ± 0.2 mmol/hr, which was significantly different from the magnesium mass balance seen with lactate fluids (− 0.1 ± 0.2 mmol/hr, p < 0.0001). There were no differences in potassium and phosphate mass balance with significant losses of phosphate (bicarbonate: − 1.7 ± 0.7 mmol/hr, lactate: − 1.7 ± 0.5 mmol/hr) and a neutral potassium balance (bicarbonate: − 0.3 ± 1.6 mmol/hr, lactate: − 0.4 ± 1.0 mmol/hr).
Figure 1. Graphic representation of electrolyte mass transfer with bicarbonate‐buffered fluids and lactate‐buffered fluids. Na = sodium, Cl = chloride, K = potassium, Mg = magnesium, Phos = phosphate. The p values refer to the difference in mass balance for chloride and magnesium with bicarbonate or lactate‐based replacement fluids.
Discussion
Isovolemic CVVH with two different replacement fluids induced significant alterations in patient mass balance for several electrolytes, which differed significantly according to the type of fluid.
Sodium and Potassium
During isovolemic CVVH, patients gained a significant amount of sodium irrespective of replacement fluid. For example, approximately 23 mmol of sodium were gained per hour. This gain occurred because the 2 L of replacement fluid infused each hour had a sodium concentration of 140 mmol/L, while the patients were generally hyponatremic, thus lowering the ultrafiltrate sodium concentration below 140 mmol/L. The clinical effects of a positive sodium balance is close to the sodium gain seen with the administration of close to 4 liters of normal saline per day and may be clinically important.
We added potassium to both replacement fluids to increase the potassium concentration from 1 mmol/L to 3.5 mmol/L. This addition seemed sufficient to prevent either gains or losses of potassium in our patients.
Chloride
Bicarbonate buffered fluid was associated with significant mass gains for chloride in the range of approximately 13 mmol/hr, which would be the equivalent of more than 2 liters of saline being administered to the patient per day. The most likely explanation for such gains is the high chloride concentration present in the bicarbonate buffered replacement fluid. It is possible that such continued gain in chloride would induce an increase serum chloride concentration over time, which could affect acid–base variables such as the anion gap and might result in a mild hyperchloremic acidosis. The more physiologic concentration of chloride in the lactate‐buffered fluid was associated with a neutral chloride mass balance.
Magnesium
There were significant magnesium losses with the bicarbonate‐based replacement fluid for an approximate daily loss of 14 mmol. This loss was most likely related to the relatively low concentration of magnesium in the replacement fluid and was not seen with the lactate buffered fluids, which contain a magnesium concentration of 0.3 mmol/L higher than the bicarbonate‐buffered fluid. Given the potential consequences of magnesium depletion and the fact that blood levels are poor indicators of total body magnesium stores or intracellular magnesium concentration, it would seem advisable to replace such magnesium losses.
Phosphate
We found that phosphate removal was approximately 1.7 mmol/hr. These observations suggest that serum phosphate concentration should be closely monitored during isovolemic CVVH and that phosphate administration is mandatory once normophosphatemia is achieved.Citation[9] The replacement dose requirements can be calculated from our observations to approximate 40 mmol/day.
This study has some limitations. First, only eight patients were studied. Some of the findings could have been influenced by individual variation, as mass transfer is dependent on the serum concentration of each solute in each patient. Nonetheless, the concentrations of solutes were not markedly abnormal and were representative of the likely values in a similar larger population of patients with ARF. Furthermore, the mass transfer of some of the electrolytes under investigation was clear and differed appreciably from one type of fluid to another, highlighting the clear differential effect of the type of commercial preparation in use. It might be argued that these observations are relatively predictable on the basis of the composition of the fluids. However, more precise empirical quantification appears desirable to better appreciate the consequences of such electrolyte shifts as well as plan a rational replacement schedule. It might also be argued that the practical implications of our observations are unclear. However, we would argue that these observations would permit a magnesium and phosphate replacement schedule, which is likely to avoid the body stores depletion of two important multifunctional electrolytes. Such a schedule may be clinically important. Our observations also have implications for commercial fluid design and suggest that more physiological chloride concentrations might be desirable as might be higher magnesium concentrations than those present in the bicarbonate replacement fluid.
In summary, we evaluated the effect of changing the type of replacement fluid on electrolyte mass balance. Isovolemic bicarbonate CVVH can cause significant gains of sodium and chloride and losses of magnesium, while lactate‐CVVH only induces sodium gains. Addition of potassium to the fluids prevents potassium losses. Calculation of magnesium and phosphate losses allows for a quantitative rational replacement schedule. Our observations might lead to the preparation of more physiological replacement fluids.
References
- Davenport A. Anionic bases for continuous forms of renal replacement therapy (CRRT) in the ICU. Intensive Care Med. 1999; 25: 1209–1211, [PUBMED], [INFOTRIEVE], [CSA]
- Heering P., Ivens K., Thumer O., Morgera S., Heintzen M., Passlik‐Deerjen J., Willers R., Strauer B. F., Grabensee B. The use of different buffers during continuous hemofiltration in critically ill patients with acute renal failure. Intensive Care Med. 1999; 25: 1244–1251, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Heering P., Ivens K., Thumer O., Brause M., Grabensee B. Acid–base balance and substitution fluid during continuous hemofiltration. Kidney Int. 1999; 56(Suppl 72)S37–S40, [CSA]
- Davenport A., Will E. J., Davison A. M. Hyperlactatemia and metabolic acidosis during hemofiltration using lactate buffered fluids. Nephron 1991; 59: 461–465, [PUBMED], [INFOTRIEVE]
- Thomas A. N., Guy J. M., Kishen R., Bowles B. M.J., Vadgama P. Comparison of lactate and bicarbonate buffered hemofiltration fluids: use in critically ill patients. Nephrol. Dial. Transplant. 1997; 12: 1212–1217, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Davenport A., Worth D. P., Will E. J. Hypochloremic alkalosis after high flux continuous hemofiltration and continuous arterio‐venous hemofiltration with dialysis. Lancet 1988; 1: 658, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bone R. C., Balk R. A., Cerra F. B., Dellinger R. P., Fein A. M., Knaus W. A., Schein R. M., Sibbald W. J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies. Chest 1992; 101: 1644–1655, [PUBMED], [INFOTRIEVE]
- Morimatsu H., Uchino S., Bellomo R., Ronco C. Continuous renal replacement therapy: does technique influence azotemic control?. Ren. Fail. 2002; 24: 645–653, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tan H. K., Bellomo R., M'Pisi D. A., Ronco C. Phosphatemic control during acute renal failure: intermittent hemodialysis versus continuous. Int. J. Artif. Organs 2001; 24: 186–191, [PUBMED], [INFOTRIEVE], [CSA]
