Abstract
Diabetic nephropathy is the common cause of end stage renal disease in diabetes mellitus. But other glomerular pathologies have been also described in diabetic patients. We described 3 cases with diabetes mellitus and other glomerular diseases. Case I: A 59‐year‐old male patient with type 2 diabetes mellitus for 4 years was evaluated for generalized edema. Physical examination showed pretibial edema and no diabetic retinopathy. The cause of nephrotic syndrome was membranoproliferative glomerulonephritis. Conservative therapy could not control the severe proteinuria and renal dysfunction. With corticosteroid and cyclophosphamide therapy partial remission was obtained. Case II: A 46‐year‐old diabetic woman was evaluated for severe proteinuria. Diabetic retinopathy was not found on her funduscopic examination. Mesangioproliferative glomerulonephritis was found on renal biopsy. Proteinuria did not regress with conservative therapy and corticosteroid and cyclophosphamide. Case III: A 48‐year‐old male patient with diabetes mellitus type 2 for 2 years was admitted to the hospital because of nephrotic syndrome and weakness. At another hospital his diagnosis with biopsy showed minimal change disease. He was treated with corticosteroid since 3 months. His renal biopsy was re‐evaluated and found amyloid deposition but not diabetic nephropathy or minimal change disease. In diabetic patients, nondiabetic nephropathy is not uncommon and it was reported as common as about 30%. In addition to therapy for diabetes mellitus these patients can need specific therapy.
Introduction
Diabetic nephropathy is the common cause of end‐stage renal disease in diabetic patients and also among the hemodialysis patients. But, in some studies, other primary glomerular diseases have been described in diabetic patients. As many as 20% of patients with diabetes mellitus type 2 evaluated for renal replacement therapy had other renal pathologies.Citation[1] In literature, co‐existence of type 2 diabetes mellitus and nondiabetic nephropathy such as membranoproliferative glomerulonephritis and amyloidosis, was uncommonly reported.Citation[2] Herein, we present three cases with diabetes mellitus type 2 and/or proliferative glomerulonephritis and amyloidosis respectively.
Case Reports
Case I
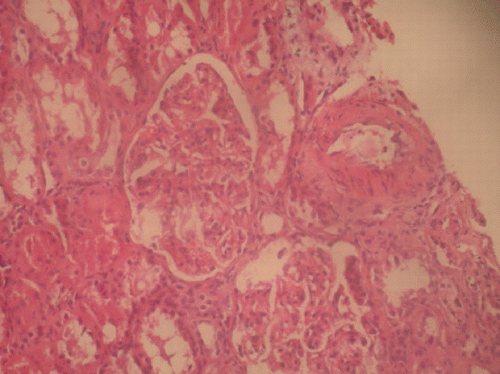
A 59‐year‐old male patient with type 2 diabetes mellitus for 4 years was admitted to the hospital because of edema weakness. Four years ago he was treated with antituberculous drugs for pulmonary tuberculosis for one year. He was afebrile and his blood pressure 130/80 mmHg and pulse 84 beats/min. Physical examination showed edema on lower extremities. Funduscopic examination did not show diabetic retinopathy. Laboratory investigations showed showed; hemoglobin 12.2 g/dL, white blood cell 17330/mm3, glucose 106 mg/dL, BUN 22 mg/dL, creatinine 1.1 mg/dL, Na 138 mEq/L, K 3.6 mEq/L cholesterol 318 mg/dL, HDL‐cholesterol 21 mg/dL, LDL‐cholesterol 277 mg/dL, triglyceride 98 mg/dL, total protein 4.8 g/dL, serum albumin 2 g/dL, AST 40 U/L, ALT 22 U/L. Erythrocyte sedimentation rate was 80 mm/h and daily proteinuria 10 g. The urine sediment contained white blood cells, granular casts and oval fat bodies. HBsAg, Anti‐HBs, Anti‐HCV, antinuclear antibody (ANA), Anti DNA were negative. Serum C3 (161 mg/dL), C4 (55 mg/dL) and serum protein electrophoresis were normal. PPD induration was 10 mm. Thorax CT showed inactive pleural calcification and fibronodular lesions on the left apex. Abdominal ultrasonography and renal venous Doppler ultrasonography were normal. Amyloidosis was not found on biopsies of rectum and renal. Renal biopsy showed membranoproliferative glomerulonephritis (). The patient was treated with enalaprilat 5 mg/day, simvastatin 10 mg/day, furosemide 40 mg × 2/week, dipyridamole 3 × 75 mg/day. NPH insulin was used for control of hyperglycemia. Nephrotic syndrome and renal function of our patient were deteriorated. Methylprednisolone 64 mg/day was begun. With progressively decreasing dose, steroid was continued 6 months. Remission was not obtained with this therapy schedule. Laboratory investigations were as follows: glucose 132 mg/dL, total protein 4.8 g/dL, serum albumin 1.8 g/dL, total cholesterol 644 mg/dL, HDL‐cholesterol l56 mg/dL, LDL‐cholesterol 500 mg/dL, triglyceride 439 mg/dL, BUN 24 mg/dL, creatinine 1.3 mg/dL and daily proteinuria 8.8 g. Pulse cyclophosphamide (500 mg per month), high dose methylprednisolone (64 mg/day) were started. Partial remission with total‐protein 6.8 g/dL, serum albumin 4.1 g/dL and daily proteinuria 2 g were obtained. Cyclophosphamide was discontinued at follow‐up period. On 5th year, angiotensin converting enzyme inhibitor, lipid lowering drug and low dose corticosteroid (in condition with severe proteinuria, 5–10 mg/day) diet, his clinical condition and laboratory findings did not changed.
Figure 1. H + E X400 expansion of glomerular tuft thickness of glomerular basal membrane and increment of mesangial cells and matrix. (View this art in color atwww.dekker.com.)
Case II
A 46‐year‐old diabetic woman was admitted to the hospital because of edema. Her medical history included diabetes mellitus type 2 for 9 years and psoriasis for 2 years.
Physical examination showed she was afebrile and had blood pressure of 110/70 mmHg, pulse of 80 beats/min and pretibial edema. There was no diabetic retinopathy on funduscopy.
Laboratory investigations were as follows: WBC 8800/mm3, HTC 38.4%, PLT 328.000/mm3, glucose 138 mg/dL, BUN 14 mg/dL, creatinine 0,7 mg/dL, Na 137 mEq/L, K 4 mEq/L, total cholesterol 406 mg/dL, HDL‐cholesterol 58 mg/dL, LDL‐cholesterol 119 mg/dL, triglyceride 404 mg/dL, total protein 4.4 g/dL, serum albumin 2.3 g/dL, ESR 50 mm/h and daily proteinuria 2.2 g.
C3 was normal (243 mg/dL, normal 80–310 mg/dL), but C4 was reduced (38.4 mg/dL normal 93–188 mg/dL). ANA, Anti‐DNA, HBsAg, Anti‐HBs and Anti‐HCV were negative. Mesangioproliferative glomerulonephritis was found on renal biopsy. A congo red stain was negative for amyloid.
The patient was treated with methylprednisolone (1 mg/kg/day), fosinopril (5 mg/day), atorvastatin 10 mg/day), dipyridamole (225 mg/day) and pulse cyclophosphamide (500 mg/m2, per month intravenously) for 10 months. But remission was not achieved. After 2 years her serum biochemistry and urinary tests were as follows: glucose 227 mg/dL, total protein 4,6 g/dL, serum albumin 2.3 g/dL, BUN 14 mg/dL, creatinine 0.9 mg/dL and daily proteinuria 4.5 g.
Case III
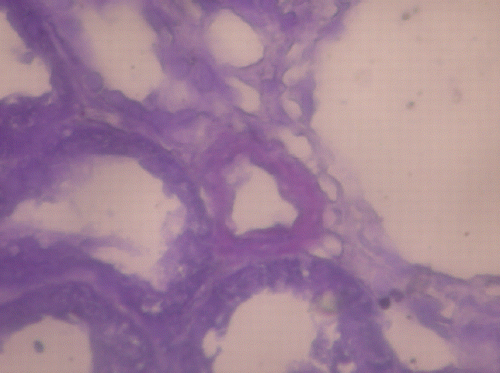
A 48‐year‐old diabetic male was admitted to our hospital because of edema and myopathy. At another hospital he was treated with glucocorticosteroid for 3 months because of biopsy proven minimal change disease (MCD). During the last 3 months, his condition worsened with the use of steroids and edema continued. He had history of diabetes mellitus type 2 for 2 years, nephrolithiasis and recurrent perianal abscess. He was afebrile and blood pressure was 80/60 mmHg. Physical examination showed edema and weakness of lower extremities. Laboratory examinations were as follows: hemoglobin 14.85 g/dL, white blood cells 14.100/mm3 and platelet 144.900/mm3, glucose 145 mg/dL, BUN 16 mg/dL, creatinine 0.8 mg/dL, Na 139 mEq/L, K 4 mEq/L total cholesterol 231 mg/dL, HDL‐cholesterol 21 mg/dL, LDL‐cholesterol 145 mg/dL, triglyceride 323 mg/dL, total protein 3.4 g/dL, serum albumin 2.3 g/dL, AST 40 U/L, ALT 50 U/L, Erythrocyte sedimentation rate and daily proteinuria were 70 mm/h and 11.25 g respectively. HBsAg, Anti HBs, Anti HCV, ANF, and Anti DNA were negative. Renal biopsy was re‐evaluated and AA amyloid depositions were found on renal and bone marrow biopsies (). Ferritin was 1140 ng/mL. Posterior anterior pulmonary graph was normal. After conservative measures and discontinued use of glucocorticosteroid his edema and weakness regressed during the 15 hospital days. The patient did not come to controls.
Figure 2. Crystal violet X400 deposition of amyloid on renal biopsy. (View this art in color atwww.dekker.com.)
Discussion
In diabetes mellitus, most causes of proteinuria are diabetic nephropathy. But sometimes nondiabetic nephropathy such as idiopathic membranous glomerulonephritis, IgA nephropathy, Henoch‐Schönlein nephritis, membranoproliferative glomerulonephritis, lupus nephritis, amyloidosis and anti‐glomerular basement membrane disease can be causes of proteinuria or renal dysfunction in diabetic patients.Citation[3] In a study the renal biopsies of 164 diabetic patients mesangioproliferative glomerulonephritis {17}, membranous glomerulonephritis {8} endocapillary proliferative glomerulonephritis {5} membranoproliferative glomerulonephritis {4} and minimal change disease {2} were reported.Citation[4] In another study including 165 diabetic patients with proteinuria, amyloidosis in 3 patients and glomerulonephritis in 25 patients were the causes of proteinuria.Citation[5] Also, there is another report of diabetic glomerulosclerosis coexistent with other renal pathologies including glomerulonephritis {14}, focal tubulointerstitial nephritis {23} and amyloidosis {1} in 38 of 136 diabetic patients.Citation[2] In our 3 patients the cause of proteinuria would be related with diabetic nephropathy or other nephropathies. Some clinical conditions such as disease duration of less than 7–10 years, macroscopic hematuria, red cell casts, white blood cell casts, no diabetic retinopathy, proteinuria without albuminuria and rapid deterioration of renal functions favor renal disease other than diabetic nephropathy and in these cases, renal biopsy should be done. As in our case the co‐existence of diabetes mellitus and membranoproliferative glomerulonephritis is very rare at 2.4%. It must be remembered that histopathologic findings of diabetic nephropathy can mimic membranoproliferative glomerulonephritis or amyloidosis.Citation[6] In our patient, histopathologic findings were prominent for membranoproliferative glomerulonephritis. While proteinuria did not respond to conservative measures, with immunosuppressive therapy proteinuria decreased to 25% of baseline level and renal function did not deteriorate in 5‐year follow‐up. In the second patient, we found mesangioproliferative glomerulonephritis. She did not respond to angiotensin converting enzyme inhibitor, regulation blood glucose and immunosuppressive treatment (glucocorticoid and cyclophosphamide). In the third patient, AA amyloid was found on renal biopsy. In this patient, low blood pressure was related to amyloidosis. However, overt diabetic nephropathy commonly accompanies high blood pressure. Also low blood pressure is not a characteristic finding for MCD. Renal amyloidosis was reported in 3 cases of 165 proteinuric diabetic patients. In literature amyloidosis was prevented or decrease of amyloidosis incidence in FMF patients with colchicine. In our patient, therapy of primary causes (perianal abscess and/or inflammatory intestinal disease) of amyloidosis and colchicine can be useful for rate limiting or stopping of progression of amyloidosis deposition.
In summary, in diabetic patients, nondiabetic nephropathy is not uncommon, and it was reported as common in about 30%. For this reason it is remembered that some diabetic patients have nondiabetic nephropathy. Blood pressure, without diabetic retinopathy, duration of diabetes are important findings for nondiabetic nephropathy. Some diabetic patients need specific therapy other than diabetes mellitus so that prognosis of patients can be changed.
References
- Magyorosi A., Ziyadeh F. N. Diabetic nephropathy. Text Book of Nephrology4th Ed., S. G. Massry, R. J. Glassock. Lippincott Williams & Wilkins, Philadelphia 2001; 874–895
- Taft J. L., Billson V. R., Nankervis A., Kincaid‐Smith P., Martin F. I. A clinical‐histological study of individuals with diabetes mellitus and proteinuria. Diabet. Med. 1990; 3: 215–221
- Ahuja T. S., Velasco A., Deiss W., Jr., Indrikovs A. J., Rajaraman S. Diabetic nephropathy with anti‐GBM nephritis. Am. J. Kidney Dis. 1998; 1: 127–130
- Chihara J., Takebayashi S., Taguchi T., Yokoyoma K., Harada T., Naito S. Glomerulonephritis in diabetic patients and its effect on the prognosis. Nephron 1986; 43(1)45–49, [PUBMED], [INFOTRIEVE]
- Ditscherlein G., Schneider W. Pathomorphological differential diagnosis of glomerular lesions in diabetics. Zentralbl. Allg. Pathol. 1986; 132(5–6)487–501, [PUBMED], [INFOTRIEVE]
- Barnes D. J., Pinto J. R., Viberti G. The patient with diabetes mellitus. Oxford Text Book of Nephrology2nd Ed., A. M. Davison, J. S. Cameron, J. P. Grünfeld. Oxford Universty Press, Oxford 1998; 723–775
