Abstract
The kidney is the main site of guanidinoacetic acid synthesis and excretion. The aim of this study was to examine whether urinary guanidinoacetic acid is a sensitive indicator for diagnosis of early‐stage gentamicin nephrotoxicity. Early‐stage renal injury was induced in rats by a single intravenous injection of 5, 10, or 30 mg/kg body weight gentamicin. Twenty‐four hours after injection all rats were killed. Blood, urine and tissue guanidino compound concentrations were determined by high performance liquid chromatography. Glycine amidinotransferase activity in tissues was assayed according to the method of Pilsum. Urinary guanidinoacetic acid excretion was decreased in 5 mg/kg gentamicin‐treated rats in comparison to that in control rats, whereas urine N‐acetyl‐β‐d‐glucosaminidase activity and β2‐microglobulin were unchanged. Guanidinoacetic acid concentration and glycine amidinotransferase activity in the kidney were significantly decreased in 5, 10, and 30 mg/kg gentamicin‐treated rats; the decreases were dose‐dependent. These results suggest that the urine guanidinoacetic acid concentration is a more sensitive indicator of renal injury than conventional indicators such as urine N‐acetyl‐β‐d‐glucosaminidase and β2‐microglobulin.
Introduction
Gentamicin has been used in the treatment of severe gram‐negative bacterial infection.Citation[1] However, use has been limited due to nephrotoxicity mainly involving the proximal tubular cells of the kidney.Citation[1] β2‐Microglobulin and N‐acetyl‐β‐d‐glucosaminidase levels have been widely used as indicators of gentamicin nephrotoxicity. These conventional indicators of renal injury, however, are not sensitive enough for early diagnosis of gentamicin nephrotoxicity.
Guanidinoacetic acid, a precursor of creatine, is synthesized from arginine in the presence of glycine by glycine amidinotransferase in the proximal renal tubules.Citation[2] Some guanidinoacetic acid synthesized in the kidney is excreted into the urine, and the rest is transported to the liver where it is methylated by guanidinoacetate‐methyltransferase to yield creatine.Citation[3] Urinary guanidinoacetic acid excretion is reported to be decreased in patients with chronic renal failure.Citation[4], Citation[5], Citation[6]
The aim of the present study was to examine whether the urinary concentration of guanidinoacetic acid is more sensitive than conventional indicators for early diagnosis of gentamicin nephrotoxicity.
Materials and Methods
Twenty‐eight male Fischer 344 rats weighing 190–210 g were used in the study. Twenty‐one of these rats were given a single intravenous injection of 5, 10, or 30 mg of gentamicin per kg body weight in 0.4 mL of 0.9% saline to induce early‐stage gentamicin nephrotoxicity. The remaining seven rats were given an injection of 0.4 mL of 0.9% saline for control. After injection each rat was housed singly in a metabolic cage and fasted but allowed free access to water. Twenty‐four‐hour urine specimens were collected. All rats were killed 24 hours after injection, and blood was collected for the determination of serum urea nitrogen (BUN), creatinine, and guanidino compound levels. The kidney and liver were removed immediately and frozen in an acetone‐dry ice mixture for assay of guanidinoacetic acid content and glycine amidinotransferase activity.
Serum was centrifuged at 1000 × g for 30 min in a Centriflo cone‐type membrane (CF‐25, Amicon Corp.). The ultrafiltrate adjusted to pH 2.2 was used for the measurement of guanidino compounds. The cut sections of renal cortex and liver were deproteinized with ice‐cold trichloroacetic acid at a final concentration of 10%. After centrifugation at 2000 × g for 10 min, trichloroacetic acid was removed from the supernatant with water‐saturated ethyl ether. The 24‐hour urine specimens were passed through 45‐µm membrane filters, and urinary creatinine excretion was determined according to the method of Jaffe.Citation[7] A part of the urine obtained by filtration was deproteinized by centrifugation in the Centriflo CF‐25 membrane at 1000 × g for 30 min. The supernatant was diluted 20‐fold with 0.01 N HCl for urinary guanidinoacetic acid determination. The amount of guanidinoacetic acid excreted was expressed as micrograms per milligram creatinine per 24 hours. The remaining urine specimens were dialyzed against tap water for 2 hours at 4°C and analyzed for N‐acetyl‐β‐d‐glucosaminidase activity and β2‐microglobulin concentration.
The guanidinoacetic acid in serum, urine, and tissues was separated by high performance liquid chromatography with the use of a Guanidinopack II (Japan Spectroscopic Co., Tokyo, Japan) eluted with 0.2 mol/L sodium citrate buffer, pH 3.0, at a flow rate of 0.5 mL/min for 10 min, and then by 0.2 mol/L sodium citrate buffer, pH 3.5, at 0.5 mL/min for 5 min. The concentrations were determined fluorometrically with the use of 9,10‐phenanthrenequinone for postlabeling.Citation[6] Fluorescence was measured (excitation at 365 nm, emission at 495 nm) with a fluorescence detector (Model FP 110‐C, Japan Spectroscopic Co.). Chromatograms were recorded on a Chromatocorder 12 (System Instrument Co., Tokyo, Japan) with a baseline display connected between the fluoro monitor and recorder, and peak areas were determined. Tissue arginine, ornithine, citrulline, and arginosuccinic acid concentrations were determined simultaneously with a high‐speed amino acid analyzer 835 (Hitachi, Ibaraki, Japan).
Glycine amidinotransferase activity in the kidney and liver was assayed using 10% homogenate, glycine, and canavanine as a substrate according to the method of Pilsum et al.Citation[8], Citation[9] Urinary N‐acetyl‐β‐d‐glucosaminidase, leucine aminopeptidase, and γ‐glutamyl transpeptidase activities were determined with m‐cresolsulfonaphthaleinyl‐N‐acetyl‐β‐d‐glucosaminide, l‐leucine p‐nitroaniline, and γ‐glutamyl p‐nitroaniline used as substrates; activity was expressed in international units (IU) or mIU/creatinine per 24‐hour urine. The urinary β2‐microglobulin level was determined by radioimmunoassay.
Data are expressed as mean ± standard error (SE). Statistical analysis was carried out by paired or unpaired Student's t‐test or analysis of variance. A p value of less than 0.05 was considered statistically significant.
Results
Serum levels of BUN and creatinine were unchanged in animals given gentamicin, suggesting that these rats were not in renal failure. The serum concentrations of guanidinoacetic acid and guanidinosuccinic acid were also unchanged in comparison to those in controls ().
Table 1. Serum Concentrations of BUN, Creatinine, Guanidinoacetic Acid, and Guanidinosuccinic Acid in the Study Rats
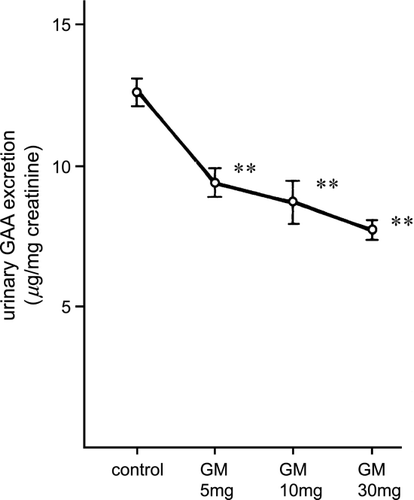
The urinary N‐acetyl‐β‐d‐glucosaminidase activity of rats injected with gentamicin at the various doses is shown in . N‐acetyl‐β‐d‐glucosaminidase in the rats given 10 or 30 mg/kg of gentamicin was significantly increased (p < 0.01, vs. control rats), whereas it was unchanged in the rats given 5 mg/kg gentamicin. Urinary β2‐microglobulin excretion was also increased significantly in the rats injected with 10 mg/kg or 30 mg/kg gentamicin (p < 0.01) but not those given 5 mg/kg. Urinary γ‐glutamyl transpeptidase and leucine aminopeptidase activities were significantly increased in 30 mg/kg gentamicin‐treated rats (p < 0.05) but not in the 5 mg/kg or 10 mg/kg gentamicin‐treated rats (). However, as shown in , urinary guanidinoacetic acid excretion was significantly decreased even in the rats treated with 5 mg/kg gentamicin (p < 0.01).
Figure 1. Urinary N‐acetyl‐β‐d‐glucosaminidase activity in rats injected with gentamicin at various doses. NAG: N‐acetyl‐β‐d‐glucosaminidase, GM: gentamicin. ** p < 0.01.
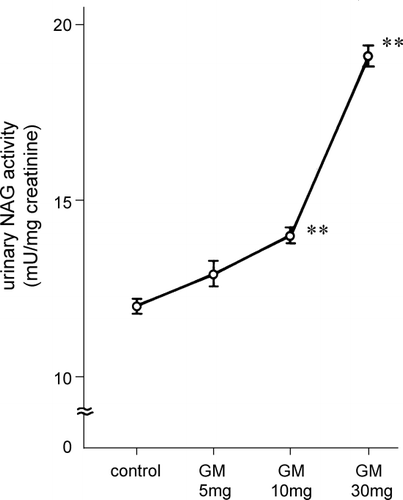
Figure 2. Urinary γ‐glutamyl transpeptidase and leucine aminopeptidase activity in gentamicin‐treated rats. γ‐GTP: γ‐glutamyl transpeptidase, LAP: leucine aminopeptidase, GM: gentamicin. * p < 0.05.
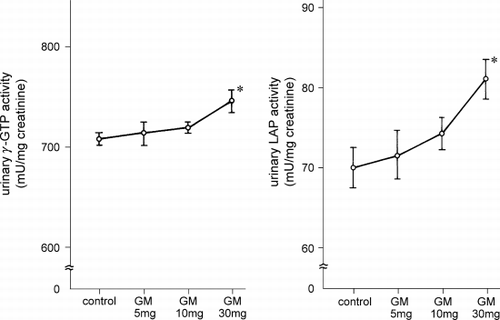
Figure 3. Urinary guanidinoacetic acid excretion in rats injected with gentamicin at various doses. GAA: guanidinoacetic acid, GM: gentamicin. ** p < 0.01.
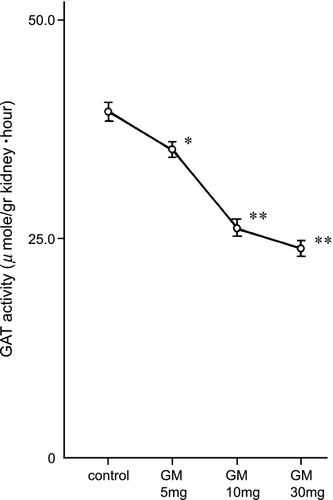
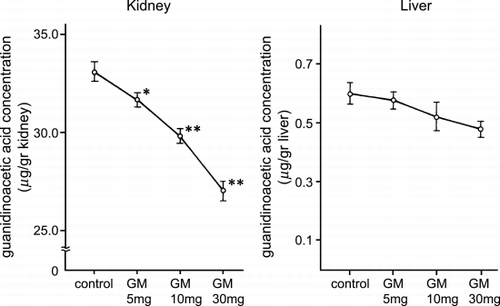
The tissue concentrations of guanidinoacetic acid are shown in . The renal guanidinoacetic acid content was significantly decreased in 5 mg/kg (p < 0.05), 10 and 30 mg/kg (p < 0.01) gentamicin‐treated rats and the decreases were dose‐dependent. The hepatic guanidinoacetic acid content decreased but not significantly. Guanidinosuccinic acid and other amino acids including arginine, citrulline, ornithine, and arginosuccinic acid in the kidney and the liver were unchanged by gentamicin administration.
Figure 4. Guanidinoacetic acid concentration in the kidney and liver. GM: gentamicin. * p < 0.05, ** p < 0.01.
Renal glycine amidinotransferase activity was significantly decreased in 5 mg/kg (p < 0.05), 10 and 30 mg/kg (p < 0.01) gentamicin‐treated rats ().
Discussion
Gentamicin is reported to induce the proximal tubular injury that results in acute renal failure.Citation[1] Morphologic changes in proximal tubule cells can be detected within hours of gentamicin administration.Citation[10] The precise mechanism underlying cell injury and cell death induced by gentamicin is unknown, but interactions with the cell membrane as well as mitochondria are likely involved.Citation[11], Citation[12]
Guanidinoacetic acid is synthesized by glycine amidinotransferase localized in the inner mitochondrial membraneCitation[2] only from arginine or canavanine and glycine in isolated rat tubules.Citation[13] We reported previously that glycine amidinotransferase is located exclusively in the first (S1) and the second (S2) portions of the proximal tubule in rats,Citation[14] where gentamicin nephrotoxicity often occurs.Citation[1] Thus, we thought it possible that the urinary guanidinoacetic acid concentration would closely reflect the proximal tubule injury induced by gentamicin administration.
Rats injected with 5 mg/kg of gentamicin showed a preferential decrease in urinary excretion of guanidinoacetic acid without any change in urinary N‐acetyl‐β‐d‐glucosaminidase activity or β2‐microglobulin concentration. These results suggest that urinary guanidinoacetic acid is more sensitive than other indicators for detecting gentamicin‐induced nephrotoxicity. The urine guanidinoacetic acid level reflects the capacity for intracellular synthesis, the urine β2‐microglobulin level reflects cellular function, and the urine N‐acetyl‐β‐d‐glucosaminidase activity reflects release of enzymes from lysosomes via cell destruction. Thus, there are differences in clinical significance of the three indicators.
The reduction in urinary guanidinoacetic acid excretion is closely associated with decreased renal synthesis of guanidinoacetic acid and decreased renal glycine amidinotransferase activity. Since guanidinoacetic acid is formed from arginine by glycine amidinotransferase in the renal tubules, it is highly likely that the decreased excretion of urinary guanidinoacetic acid is the result of the decreased renal activity of glycine amidinotransferase, probably inhibited by relatively small doses of gentamicin.
In conclusion, urinary guanidinoacetic acid is a sensitive and specific indicator for early‐stage gentamicin nephropathy.
References
- Humes H. D., Weinberg J. M., Knauss T. C. Clinical and pathophysiologic aspects of aminoglycoside nephrotoxicity. Am. J. Kidney Dis. 1982; 2: 5–29, [PUBMED], [INFOTRIEVE]
- Margi E., Baldoni G., Grazi E. On the biosynthesis of creatine. Intramitochondrial localization of transamidinase from rat kidney. FEBS Lett. 1975; 55: 91–93, [CROSSREF]
- Gerber G. B., Gerber G., Koszaika T. R., Miller L. L. The rate of creatine synthesis in the isolated, perfused rat liver. J. Biol. Chem. 1962; 237: 2246–2250, [PUBMED], [INFOTRIEVE]
- Sasaki M., Takahara K., Natelson S. Urinary guanidinoacetate/guanidinosuccinate ratio: an indicator of kidney dysfunction. Clin. Chem. 1973; 19: 315–321, [PUBMED], [INFOTRIEVE]
- Marescau B., Nagels G., Possemiers I., De Broe M. E., Because I., Billiouw J. M., Lornoy W., De Deyn P. P. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 1997; 46: 1024–1031, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tanaka A., Takahashi Y., Mizokuchi M., Shimada N., Koide H. Plasma, urinary, and erythrocyte concentrations of guanidino compounds in patients with chronic renal failure. Ren. Fail. 1999; 21: 499–514, [PUBMED], [INFOTRIEVE], [CSA]
- Jaffe M. Uber den niedershlag, welchen pikrinsaure in normalem harn erzeugt und uber eine neue reaktion des kreatinins. Hoppe‐Seyler Z. Physiol. Chem. 1886; 10: 391, [CSA]
- Pilsum J. F.V., Berman D. A., Wol E. A. Assay and some properties of kidney transaminidase. Proc. Soc. Exp. Biol. Med. 1957; 95: 96–100
- Pilsum J. F.V., Olsen B., Taylor D., Rozycki T., Pierce J. C. Transamidinase activities, in vitro, of tissues from various mammals and from rat fed protein‐free, creatine‐supplemented and normal diets. Arch. Biochem. Biophys. 1963; 100: 520–524, [CROSSREF]
- Silverblatt F. J., Kuehn C. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int. 1979; 15: 335–345, [PUBMED], [INFOTRIEVE]
- Simmons C. F., Jr., Bogusky R. T., Humes H. D. Inhibitory effects of gentamicin on renal mitochondrial oxidative phosphorylation. J. Pharmacol. Exp. Ther. 1980; 214: 709–715, [PUBMED], [INFOTRIEVE]
- Mela‐Riker L. M., Widener L. L., Houghton D. C., Bennett W. M. Renal mitochondrial integrity during continuous gentamicin treatment. Biochem. Pharmacol. 1986; 35: 979–984, [CROSSREF]
- Takeda M., Kiyatake I., Koide H., Jung K. Y., Endou H. Biosynthesis of guanidinoacetic acid in isolated renal tubules. Eur. J. Clin. Chem. Clin. Biochem. 1992; 30: 325–331, [PUBMED], [INFOTRIEVE]
- Takeda M., Koide H., Jung K. Y., Endou H. Intranephron distribution of glycine–amidinotransferase activity in rats. Renal. Physiol. Biochem. 1992; 15: 113–118, [PUBMED], [INFOTRIEVE]