Abstract
Immature acerola juice was dehydrated by spray drying, using as encapsulating material maltodextrin DE25, arabic gum, or a mixture of both in different proportions. A constant ratio of 1:1 was kept between juice solids and encapsulating material. The effect of encapsulation materials on water sorption, glass transition, and physical properties of encapsulated immature acerola juice was investigated. The monolayer moisture of the encapsulated juices, calculated according to the GAB theory, varied from 5.11 to 5.73g H2O/100g of solids (25°C). The glass transition temperature (Tg) of maltodextrin and gum arabic varied from 60 (aw 0.33) to 38°C (aw 0.54), and from 62 (aw 0.33) to 42.6°C (aw 0.54), respectively. The addition of juice to the encapsulating materials decreased the Tg of the juice powder to 39.5–41.3°C (aw 0.33) and 1.84–8.05°C (aw 0.54), but no marked differences were found among the juice powders. The critical aw, i.e., the point of onset of physical alterations in the encapsulated materials, was higher than the corresponding monolayer values. Stickiness was observed at temperatures close to Tg, and collapse occurred at temperatures of 20°C or more above the Tg. Maltodextrin DE25 and gum arabic offered equivalent contributions to the stability of the system.
INTRODUCTION
Acerola (Malpighia emarginata DC), a fruit of the Malpighiaceae family, is native to the Antilles, in northern South America and in Central America. Acerola has been cultivated in Brazil since 1956,[Citation1] and its juice has enjoyed widespread acceptance in the world market, especially in Japan and the USA, where it is used as a natural source of vitamin C in mixtures with a variety of products, and with wide possibilities for further use.[Citation2] Acerola is known for its high vitamin C content, ranging from 1000 to 2500 mg vitamin C/100g pulp.[Citation3] Acerola is regarded as one of the best natural sources of this vitamin and, in human trials, has been shown to be a better source of bioavailable vitamin C than synthetic vitamin C.[Citation4] The vitamin C content of acerola decreases by 30-50% during maturation.[Citation5,Citation6] Thus, it is of interest to use dehydrated, immature fruit juice as a way of enhancing its natural vitamin C content and increasing its potential as a functional food source.
Dehydration is an alternative process for fruit juices conservation and opens new possibilities for their use in different food formulations. However, spray drying of fruit juices and other products with a high sugar content presents technical difficulties because of their hygroscopicity and thermoplasticity at high temperatures and humidities.[Citation7] For that reason, addition of maltodextrin, as well as other substances such as pectins and gums, has been used in the production of powder juices.[Citation8,Citation9] Encapsulation technology in food processing involves the coating of ingredients in order to decrease product reactivity with the environment (light, water, and oxygen), and to reduce the release of core material.[Citation10] In systems such as fruit juices, the encapsulated product consists of a homogeneously blended matrix of the polymer entrapping the core, sometimes referred to as “entrapped” ingredients.[Citation11] Spray drying is the most frequently used method for encapsulation in the food industry.[Citation11] Coating substances are basically film-forming materials and include a wide variety of natural and synthetic polymers.[Citation12,Citation13] The choice of the encapsulating agent depends on the chemical and physical properties of the product to be encapsulated, on the process used to make the microcapsules, and on the properties desired for the encapsulate.[Citation14,Citation15]
Maltodextrins consist of β-D-glucose units linked mainly by glycosidic bonds (1→4) and are usually classified according to their dextrose equivalency (DE). The DE of a maltodextrin determines its reducing capacity and is inversely related to its average molecular weight.[Citation16] Maltodextrins are mainly used in materials that are difficult to dry— such as fruit juices, flavorings, and sweeteners[Citation17]—and to reduce stickiness, thereby improving the product stability.[Citation8, Citation9,Citation18] Gum arabic is a complex heteropolysaccharide with a highly ramified structure, with a main chain formed of D-galactopyranose units joined by β-D glycosidic bonds (1→3). Side chains with variable chemical structures formed from D-galactopyranose, L-rhamnose, L-arabinofuranose, and D-galacturonic acid are linked to the main chain β-(1→6) bonds.[Citation16] Gum arabic has been the most widely used encapsulating material in microencapsulation by spray drying, mainly because of its good emulsifying capacity and low viscosity in aqueous solution, which aids the spray drying process. In addition, it provides good retention of volatile substances and confers effective protection against oxidation.[Citation19,Citation20]
Water sorption isotherms are useful thermodynamic tools for predicting the interactions of water and food components. They provide information for assessing food processing operations such as drying, packaging, and storage.[Citation21,Citation22] Such data can be used to select appropriate storage conditions and packaging systems that optimize product characteristics.[Citation22] The characteristics of water sorption, as well as most other interactions of food with water, are defined by the composition of the solid part, mainly carbohydrates and proteins. The sorption properties can also be affected by structural transformations and phase transition, both of which are time-dependent phenomena. The glass transition temperature (Tg ) can be defined as the temperature at which an amorphous system changes from a glassy to a rubbery state and constitutes a reference temperature that relates the physical properties of foods with water content and temperature.[Citation18,Citation23,Citation24,Citation25,Citation26,Citation27] Typical structural alterations occurring in amorphous foods stored at temperatures above Tg include agglomeration, stickiness, collapse and crystallization.[Citation23,Citation24] When the transition occurs, the rate of the deteriorative reactions is affected; therefore, knowledge of the sorption properties is extremely important for predicting the physical state of a food.[Citation23,Citation26] Thus, water activity and Tg are important parameters for controlling the behavior of a food and its deterioration during processing and storage.[Citation25] Maltodextrin is a widely used encapsulating agent that improves system stability during storage by increasing Tg .[Citation25,Citation26] Although gum arabic is a commonly used encapsulating agent, its contribution to the stability of dehydrated and/or encapsulated food has not been extensively studied. The aim of this work was to examine the effect of different encapsulating materials on water sorption and Tg of encapsulated immature acerola juice. Alterations in the physical properties of the encapsulated material caused by moisture sorption were also evaluated.
MATERIAL AND METHODS
Concentrated immature acerola juice was provided by the company Anidro S/A (Botucatu, SP, Brazil) and showed the following characteristics: 21% soluble solids, 4.9g/100g of vitamin C, 15.8/100g of sugars, 9.6 mg/g total phenolic compounds, and pH 7.0. Encapsulating materials were maltodextrin DE 25, donated by Corn Products (Brazil), and analytical grade gum arabic (Synth, Brazil). All other reagents were of analytical grade.
Encapsulation of Immature Acerola Juice
The encapsulating materials—gum arabic (G) and maltodextrin (M)—were added directly to the concentrated immature acerola juice to give a total of 50% soluble solids (25% from juice plus 25% from encapsulating materials). shows the different formulations of the encapsulating materials. Drying was done in a laboratory scale spray dryer (model SD-04 Lab-Plant Limited, Huddersfield, England, UK), with a 1 mm diameter injector nozzle and an air pressure of 5×104 Pa. Based on preliminary trials, the processing conditions used were air inlet temperature of 120°C, exit temperature of 80 to 82°C, and liquid flow rate of 3-3.5 ×10−5m3/s.
Table 1 Formulations of the encapsulated materials
Characterization of the Encapsulated Material
Moisture content was determined by the oven method.[Citation28] Water activity (aw ) was determined by means of a model CX-2T Aqualab (Decagon Devices Inc., Pullman, Washington, DL, USA). Measurements were done in triplicate, at 25 °C.
Sorption Isotherms
Sorption isotherms were determined gravimetrically at 25, 35, and 45 °C. Triplicate vials containing about 1g of powder were transferred to desiccators over saturated salt solutions of LiCl, MgCl2, K2CO3, Mg2NO3, NaCl, and BaCl2, to provide relative humidities of 11%, 32%, 43%, 52%, 75%, and 89%, respectively. Samples were weighed every five days until a weight change below 0.001g was recorded on two consecutive weighings, at which point the samples were assumed to be at equilibrium (generally within 20 days). After reaching equilibrium, the color and physical appearance of the samples were observed. The recipients where the samples were equilibrated at different relative humidities (11-89%) and temperatures (25, 35, and 45°C) were tapped sharply against a hard surface to check whether the powder: (a) was flowing freely; or (b) had agglomerated or caked, with particles failing to separate, appearing as clumps even after shaking; or (c) had collapsed, with particles forming a block, with loss of structure.[Citation26,Citation27] Adjustments of the mathematical model and calculation of the Guggenheim-Anderson-de Boer (GAB) equation parameters[Citation29] were done with help of the Water Analyzer Series software (Isotherm/BET/GAB Program, version 2.05 p.) for Macintosh (St. Paul, MN, USA).
Differential Scanning Calorimetry
Calorimetric measurements were carried out with a TA Instruments Universal v2.3c calorimeter. The equipment was calibrated by measuring the melting temperature of indium (156.6°C) and water (0°C), according to the manufacturer's instructions. The glass transition temperature (Tg ) was determined from the midpoint of heat capacity change observed at a heating rate of 10°C/min. Samples (10mg) were placed in aluminum DSC pans weighed in uncoated DSC. Samples were allowed to equilibrate over saturated salt solutions and were then hermetically sealed for analysis. Two runs per sample were completed, the first one to eliminate the relaxation enthalpy that could interfere with determination of the glass transition temperature. The analyses were done at least in duplicate. The concentrated juice was lyophilized before DSC analysis.
RESULTS AND DISCUSSION
shows the initial moisture content and aw of encapsulating materials, concentrated juice from immature acerola, and juice encapsulated with different encapsulating formulations. The aw of the encapsulated samples varied from 0.199 to 0.292, typical values for dehydrated foods, corresponding to 4.4 to 6.8g of H2O/100 g of sample. All the water sorption isotherms of the encapsulated acerola juice with different formulations showed similar sigmoidal curves (), characteristic of most foods.[Citation30] As the initial aw of the samples was 1.99–2.92, desorption occurred at 11% RH, absorption being dominant at higher RH. The equilibrium moisture increased very slowly with increasing aw up to 0.52, beyond which there was a steep rise in the moisture of all samples. In this region, water had a predominant influence on the powder stability because, being in the form of free molecules, it can dissolve constituents, resulting in acceleration of the undesirable reactions.[Citation31,Citation32] Other dehydrated products, such as powdered soy sauce and miso,[Citation33] as well as encapsulated products such as microcapsules of essential orange oil,[Citation34] are reported to show similar behavior.
Figure 1 GAB sorption isotherms experimental data the encapsulated juice from immature acerola with different formulation at 25º, 35º, 45ºC. The arrows indicate the of the samples (1) free flowing powder; (2) free flowing property reduced (stickiness); (3) lump formatioins (caking); (4) loss of structure (collapse); (5) browning and hard structure; (6) high viscosity liquid. Legend : ♦ 20%M; j 15%M + 5%G; ▴ 10%M + 10%G; ⊠ 5%M + 15%G; • 20%G.
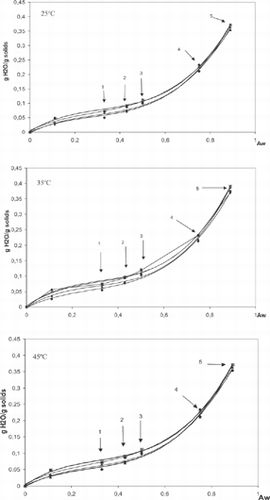
Table 2 Initial moisture content and water activity () of encapsulated immature acerola juice with different formulations
The equilibrium moisture of the samples decreased with increasing temperature. This trend may be explained by sorption thermodynamics, since an increase in temperature creates unfavorable conditions for the adsorption of water.[Citation34] Some studies[Citation32,Citation34] have reported an inversion of the effect of temperature in samples with an aw around 0.7 caused by an increase in the solubility of sugars in water. This phenomenon was also observed for the encapsulated juice. The parameters calculated for the GAB equation are shown in . The average error (P), expressing the difference between the calculated model and the experimental data, was <10%, except for formulation 15%M+5%G and the formulation 20%M at 45°C, which were 10.2% and 10.6%, respectively. In these two cases, probably some deviation occurred due to liquefaction of samples at high temperature and RH. Monolayer values (Xm) varied from 0.0452 (20%M at 45°C) to 0.0573 g H2O/100g solids (15%M+5%G at 15°C). Desobry et al.[Citation36] found similar monolayer values (0.05 to 0.065g of H2O/g of solids) for betacarotene encapsulated with maltodextrin DE25. Roos[Citation25] reported 0.0491g of H2O/g of solids (BET) for maltodextrin DE25. Asheri[Citation34] obtained lower monolayer values (between 0.018 and 0.031g of H2O/g of solids) for essential orange oil encapsulated with a mixture of maltodextrin, gum arabic, and Capsul®.
Table 3 GAB (Xm , C, K) parameters fitted to the isotherms of moisture sorption of encapsulated juice from immature acerola
Energy constants C and K influence the sigmoidal shape of the isotherms. Constant C determines the more or less pronounced form of the “knee” in the lower aw range, whereas constant K determines the isotherm profile in the higher aw range. All isotherms showed similar shape; hence, no differences were expected amongst the GAB constants. The K values did not show variation among the samples (∼1); they were higher than those reported by Timmermann et al.[Citation37] for various food product (0.62 to 0.88), and similar to those for encapsulated beta carotene in maltodextrin matrixes.[Citation36] This high value of K produces a marked increase in the isotherm slope in the higher aw range.[Citation37] Although C values ranged from 2.5 to 98, no marked differences amongst isotherm shapes were observed in the lower aw range. The shape and position of the isotherm are known to be influenced by sample composition, physical structure (crystalline or amorphous), and pretreatment;[Citation30] this study, however, found similar sorption isotherms for samples of different composition.
The arrows in indicate the aw at which the physical characteristics of encapsulated materials changed after reaching equilibrium at different relative humidities and temperatures. Samples with an aw < 0.33 stored at 25 and 35°C remained as free flowing powders. Stickiness was observed in samples with an aw of 0.43, and caking in samples with an aw of 0.5. Collapse and liquefaction were observed in samples stored at relative humidities >75%. Consistency of these samples varied from hard to plastic to gluey as aw increased. Samples stored at 45°C showed the same alterations in their physical properties, but at lower relative humidities. Exposure to high temperature or dissolution of soluble compounds by wetting leads to plasticization, causing formation of liquid bridges,[Citation28] one of the predominant mechanisms in the caking of foods. A similar behavior was described for fish protein hydrolysates[Citation38,Citation39,Citation40] and for onion powder.[Citation21] The temperature at which color change (browning) occurs depends on the RH. At 25°C browning occurred at RH51%, whereas at 35-45°C, color changes were observed even in samples stored under low RH (33%). Formulations with predominance of maltodextrin showed less browning than those with a predominance of gum arabic. The protein fraction of the gum arabic may have favored the Maillard reaction.
Agglomeration and caking of food powders during storage are deleterious phenomena that limit the use of powders. The main cause of caking and agglomeration is water-induced plasticization of the particle surface. These physical changes were successfully explained and predicted by the glass transition concept on the basis of Tg as a reference temperature.[Citation26] Such physical transformations have been observed to occur above Tg .[Citation38, Citation41] Tg values for maltodextrin, gum arabic, concentrated acerola juice, and encapsulated juice, are shown in . Maltodextrin and gum arabic showed similar Tg values at aw in the range of 0.33 to 0.51. Addition of gum arabic and/or maltodextrin to the concentrated juice (Tg =0.5°C, aw =0.43) resulted in encapsulated materials with higher Tg than the juice alone. Since Tg values increase with increasing molecular weight, high molecular weight amorphous polymers, such as maltodextrins, were used to raise the Tg of the fruit juice powders and improve product stability.[Citation18,Citation42] At an aw of 0.43, the formulation 20%G had the highest Tg (34°C), while the other encapsulated materials had Tg ranging from 25.7 to 27.1°C. Regardless of the encapsulating material formulation, all juice powders showed similar Tg at the same aw . The Tg of a mixture of various compatible components (including water) is a nonlinear function of the the Tg of individual components.[Citation43] Gordon and Taylor[Citation44] and Couchmann & Karasz[Citation45] proposed a mathematical relationship for determining the Tg of a mixture. The Gordon and Taylor equation relates the Tg of miscible blends to the fractional concentration of their constituents, including water, and is typically applied either to predict the influence of moisture content on the Tg , or to estimate the Tg of a binary mixture.[Citation23, Citation24,Citation43] Since maltodextrin DE25 and gum arabic had similar Tg values in the aw range studied and all encapsulated samples contained the same amount of solids derived from the juice (44%) and from the encapsulating agents (56%), as well as a similar water content (), the Tg of the encapsulated juices were expected to be similar, as was indeed the case.
Table 4 Glass transition temperature (Tg) of gum arabic (G), maltodextrin DE 25 (M), and encapsulated juice from immature acerola juice with different Aw
Physical alterations occurred in the samples () at an aw greater than that of the monolayer value (). This moisture level was, indeed, the level at which the powders keep very well for a long period (>1 year). Samples with aw 0.33, Tg 39-45°C, were free flowing powders. At ambient temperature (25°C) the samples with aw 0.43 showed stickiness, as did the samples with an aw of 0.33 stored at 45°C. Even samples stored in the vicinity of their Tg showed the initial stages of caking. Samples with an aw of 0.52 and Tg below room temperature showed marked transformation of their physical properties, the severity of which increased with temperature. These results agreed with Roos,[Citation25] who found that BET monolayer values for maltodextrins of different DE were significantly lower than the critical moisture content, i.e., the point at which physical alterations in the materials started.[Citation46] However, according to Roos,[Citation18,Citation25] the critical aw and moisture contents were those that depressed the Tg to room temperature or below it, and, therefore, structural changes such as stickiness, collapse and crystallization of amorphous foods are related to water content and water activity.[Citation23] Collapse of the encapsulated juice samples was observed at 10 to 20°C above the Tg . Roos & Karel[Citation18] observed collapse of maltodextrins (DE 25-10) at temperatures 40 to 70°C above the Tg .
CONCLUSIONS
Immature acerola juice encapsulated with maltodextrin DE25, gum arabic, or a mixture of both showed similar sorption isotherms. The critical aw range, in which physical transformations occurred in the encapsulated samples, was 0.33-0.43, depending on the storage temperature. Physical alterations occurred at aw greater than those of the monolayer values. Maltodextrin DE25 and gum arabic showed similar Tg values at aw values of 0.33-0.52, as did the encapsulated materials. Stickiness was observed at temperatures close to the Tg and collapse at < 20°C above the Tg . Maltodextrin DE25 and gum arabic contributed similarly to the stability of the system.
ACKNOWLEDGMENT
The authors are grateful to FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for their financing of this project, and to CNPq (Conselho Nacional Desenvolvimento Científico e Tecnológico) for the grant to the author Righetto.
REFERENCES
- Oliva , P.B. , Menezes , H.C.de and Ferreira , V.P. 1996 . Estudo da estabilidade do néctar de acerola . Ciência e Tecnologia de Alimentos , 16 ( 3 ) : 228 – 232 .
- Korgo , A. 1996 . Development of consumption and raw materials: till today and in the future . Fruit Processing , 6 ( 12 ) : 478 – 481 .
- Carvalho , R.I.N. de and Manica , I. 1994 . Influência de estágios de maturação e condições de armazenamento na conservação da acerola (Malpighia glabra L.) . Pesquisa Agropecuária Brasileira , 29 ( 5 ) : 677 – 680 .
- Hwang , J. , Hodis , H.N and Sevanian , A. 2002 . Soy and alfalfa extracts become potent low-density lipoprotein antioxidants in the presence of acerolacherry extracts . Journal of Agricultural and Food Chemistry , 49 : 308 – 314 . [CROSSREF]
- Itoo , S. , Mitsuco , A. and Ishihata , K. 1990 . Comparison of ascorbic acid content in acerola fruit from different production region depend on degree of maturity, and stability by processing . Nippon Shokuhin Kogyo Gakkaishi , 37 ( 9 ) : 726 – 729 .
- Vendramini , A.L. and Trugo , L.C. 2000 . Chemical composition of acerola fruit (Malpighia puncifolia L.) at three stages of maturity . Food Chemistry , 71 : 195 – 198 . [CROSSREF]
- Borges , S.V. , Reis , A.L.S.H. , Jorge , E.C. , Pinto , P.R. and Oliveira , V.M. 2002 . Jugo de frutas tropicales deshidratado por “spray-drying” Alimentaria , 12 : 125 – 130 .
- Bhandari , B.R. , Snoussi , A. , Dumoulin , E.D. and Lebert , A. 1993 . Spray drying of concentrated fruit juices. . Drying Technology , 11 ( 5 ) : 1081 – 1092 .
- Bhandari , B.R. , Datta , N. and Howes , T. 1997 . Problems associated with spray drying of sugar-rich foods . Drying Technology , 15 ( 20 ) : 671
- Dziezak , J.D. 1998 . Microencapsulation and encapsulated ingredients . Food Technology , 42 ( 4 ) : 136 – 148 .
- Uddin , M.S. , Hawlader , M.N.A. and Zhu , H.J. 2001 . Microencapsulation of ascorbic acid: effect of process variables on product characteristics . Journal of Microencapsulation , 18 ( 2 ) : 199 – 201 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Shahidi , F. and Han , X.Q. 1993 . Encapsulation of food ingredients . Critical Review in Food Science and Nutrition , 33 ( 6 ) : 501 – 547 .
- Runge , F.E. 2001 . “ Multiple-core encapsulation ” . In Microencapsulation of Food Ingredients , Edited by: Vilstrup , P. 133 – 141 . Leatherhead : Leatherhead Publishing .
- Jackson , L.S. and Lee , K. 1991 . Microencapsulation and the food industry . Lebensmittel-Wissenchaft Technologie Food Science , 24 ( 4 ) : 289 – 297 .
- Marquardt , U. 1992 . The use of microencapsulated ingredients and additives in food . Food Ingredients International , 4 : 17 – 19 .
- BeMiller , J.N. and Whistler , R.L. 1996 . “ Carbohydrates ” . In Food Chemistry, , 3rd , 157 – 224 . New York : Marcel Dekker .
- Reineccius , G.A. “ Multiple-core encapsulation: the spray drying of food ingredients ” . In Microencapsulation of Food Ingredients , 151 – 185 . Leatherhead : Leatherhead Publishing .
- Roos , Y. and Karel , M. 1991 . Applying state diagrams to food processing and development . Food Technology , 45 ( 12 ) : 66 – 71, 107 . [PUBMED] [INFOTRIEVE]
- Rosemberg , M. , Kopelman , I.J. and Talmon , Y. 1990 . Factors affecting retention in spray-drying microencapsulation of volatile materials . Journal of Agricultural and Food Chemistry , 38 : 1288 – 1294 . [CROSSREF]
- Rennecius , G.A. 1991 . Carbohydrates for flavor encapsulation . Food Technology , 46 ( 3 ) : 144 – 152 .
- Debnath , S. , Hemavathy , J. and Bhat , K.K. 2002 . Moisture sorption studies on onion powder . Food Chemistry , 78 : 479 – 482 . [CROSSREF]
- Labuza , T.P. , Kaanane , A. and Chen , J.Y. 1985 . Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods . Journal of Food Science , 50 : 385 – 391 .
- Roos , Y.H. 1995 . Phase Transitions in foods; , San Diego : Academic Press .
- Aguilera , J.M. , Del Valle , J.M. and Karel , M. 1995 . Caking phenomena in amorphous food powder . Trends in Food Science and Technology , 6 ( 5 ) : 149 – 155 . [CROSSREF]
- Roos , Y. 1993 . Water activity and physical state effects on amorphous food stability . Journal of Food Processing and Preservation , 16 : 433 – 447 .
- Chuy , L.E. and Labuza , T.P. 1994 . Caking and stickness of dairy-based food powders as related to glass transition . Journal of Food Science , 59 ( 1 ) : 43 – 46 .
- Aguilera , J.M. and Del Valle , J.M. 1995 . “ Structural changes in low moisture food powders ” . In Food Preservation by Moisture Control , Edited by: Brabosa-Cánovas , G.V. and Welti-Chaves , J. 675 – 695 . Lancaster, PA : Technomic Pub. Co .
- AOAC. 1984 . Official Methods of Analysis, , 12th , Washington, DC : AOAC International .
- Van Den Berg , C. and Bruin , S. 1981 . “ Water activity and its estimation in food systems: Theoretical aspects ” . In Water Activity: Influences on Food Quality , Edited by: Rockland , L.B. and Stewart , G.F. 1 – 61 . New York : Academic Press .
- Fennema , O.R. 1996 . “ Water and ice ” . In Food Chemistry, , 3rd , Edited by: Fennema , O.R. 157 – 224 . New York : Marcel Dekker .
- Labuza , T.P. 1984 . Moisture Sorption: Practical Aspects of Isotherm Measurement and Use , St. Paul, MN : American Association of Cereal Chemists .
- Tsami , E. , Marinos-Kouri, D. and Maroulis , Z.B. 1990 . Water sorption isotherms of raisins, currants,figas prunes, and apricots . Journal of Food Science , 55 : 1594 – 1597 .
- Hamano , M. and Sugimoto , H. 1978 . Free flowingness of powdered soy sauce and miso . Journal of Food Processing and Preservation , 2 : 185 – 196 .
- Asheri , D.P.R. 1999 . Estudo das características de adsorção de água e da estabilidade das microcápsulas de óleo essencial de laranja na seleção de material de parede . Ciência e Tecnologia de Alimentos , 19 ( 3 ) : 1 – 17 .
- Iglesias , H.A. and Chirife , J. 1982 . Handbook of Food Isotherms , New York : Academic Press .
- Desobry , S.A , Netto , F.M. and Labuza , T.P. 1997 . Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation . Journal of Food Science , 62 ( 12 ) : 1158 – 1162 .
- Timmermann , E.O. , Chirife , J. and Iglesias , H.A. 2001 . Journal of Food Engineering , 48 : 19 – 31 . [CROSSREF]
- Aguilera , J.M. , Levi , G. and Karel , M. 1995 . Effect of water content on the glass transition and caking of fish proteins hydrolysates . Biotechnology Progress , 9 : 651 – 654 . [CROSSREF]
- Netto , F.M. , Desobry , S.A. and Labuza , T.P. 1998 . Effect of water content on the glass transition,caking, and stickiness of protein hydrolysates . International Journal of Food Properties , 1 : 141 – 161 .
- Jardim , D.C.P. , Cândido , L.M.B. and Netto , F.M. 1999 . Sorption isotherms and glass transition temperatures of fish protein hydrolysates with different degrees of hydrolysis . International Journal of Food Properties , 2 : 227 – 242 .
- Champion , D. , Le Meste , M. and Simatos , D. 2000 . Towards an improved understanding of glass transition and relaxation in foods: molecular mobility in the glass transition range . Trends in Food Science and Technology , 11 : 41 – 55 . [CROSSREF]
- Ross , Y and Karel , M. 1991 . Water and molecular weight effects on glass transition in amorphous carbohydrate solution . Journal of Food Science , 56 : 1676 – 1681 .
- Bhandari , B.R. and Howes , T. 1999 . Implication of glass transition for the drying and stability of dried foods . Journal of Food Engineering , 40 : 71 – 79 . [CROSSREF]
- Gordon , M. and Taylor , J.S. 1952 . Non-crystalline copolymers . Journal of Applied Chemistry , 2 : 493 – 500 .
- Couchman , P.R. and Karasz , F.E. 1978 . A classical thermodynamic discussion of the effect of composition on glass-transition temperatures . Macromolecules , 11 ( 1 ) : 117 – 119 . [CROSSREF]
- Labuza , T.P. , Tannembaum , S.R. and Karel , M. 1970 . Water content and stability of low moisture and intermediate-moisture foods . Food Technology , 24 : 542 – 544 .
