Abstract
The development of biomaterials with intrinsic antioxidant properties could represent a valuable strategy for preventing the onset of peri-implant diseases. In this context, quercetin, a naturally occurring flavonoid, has been entrapped at different weight percentages in a silica-based inorganic material by a sol–gel route. The establishment of hydrogen bond interactions between the flavonol and the solid matrix was ascertained by Fourier transform infrared spectroscopy. This technique also evidenced changes in the stretching frequencies of the quercetin dienonic moiety, suggesting that the formation of a secondary product occurs. Scanning electron microscopy was applied to detect the morphology of the synthesized materials. Their bioactivity was shown by the formation of a hydroxyapatite layer on sample surface soaked in a fluid that simulates the composition of human blood plasma. When the potential release of flavonol was determined by liquid chromatography coupled with ultraviolet and electrospray ionization tandem mass spectrometry techniques, the eluates displayed a retention time that was 0.5 min less than quercetin. Collision-activated dissociation mass spectrometry and untraviolet-visible spectroscopy were in accordance with the release of a quercetin derivative. The antiradical properties of the investigated systems were evaluated by DPPH and ABTS methods, whereas the 2,7-dichlorofluorescein diacetate assay highlighted their ability to inhibit the H2O2-induced intracellular production of reactive oxygen species in NIH-3T3 mouse fibroblast cells. Data obtained, along with data gathered from the MTT cytotoxicity test, revealed that the materials that entrapped the highest amount of quercetin showed notable antioxidant effectiveness.
1. Introduction
In recent years, research in the field of biomaterials endowed with high bioactivity and biocompatibility has given increased attention to the preparation of dental and/or orthopedic devices and implants [Citation1–Citation3]. New synthetic strategies have been developed to define a broad and durable application of these devices, which respond well to a more demanding request by the end user to combine the concepts of health and aesthetics, especially in the field of dentistry [Citation4–Citation7].
The promising chemical and physical properties of different biomaterials have been widely investigated [Citation8, Citation9], and detailed knowledge about their potential toxicities has also been provided [Citation10–Citation14]. Nevertheless, diseases resulting from failures of an implant system are still continuously reported. An example is peri-implantitis, which takes hold in the oral cavity as a result of a multifactorial process where the dominant role seems to be the overproduction of reactive oxygen species (ROS) [Citation15]. At the cellular level, this imbalance between the production of reactive chemical species with radical and nonradical natures causes an oxidative stress condition. Oxidative stress is defined as an uncontrolled process [Citation16] by which the massive production of reactive species is conjugated to the damage they can cause to biological molecules (e.g., DNA, proteins, carbohydrates, and lipids). The sustained damage by ROS results in harmful consequences at the cellular, tissue, and systemic levels. It has been broadly demonstrated that this condition is involved in the establishment of some chronic and degenerative diseases (e.g., cancer and neurodegenerative diseases), cardiovascular diseases, and in oral cavity pathologies such as periodontitis. As for periodontal disease [Citation17], peri-implantitis is triggered by gram-negative, anaerobic, or microaerophilic bacteria and by the establishment of an inflammation outbreak capable of inducing the formation of ROS. Once formed, the ROS stimulate the production of proinflammatory cytokines, thus triggering a vicious circle [Citation18]. Some evidence suggests that the total antioxidant capacity of saliva undergoes a significant decrease in the peri-implantitis condition; the concentration of uric and ascorbic acids—the main metabolites with antioxidant activity—appears to be strongly reduced [Citation19]. In this context, biomaterials research that can counter the damaging effects of oxidative stress could represent a valuable and useful tool for therapies that target these medical problems. Indeed, the hypothesis of counteracting the onset of peri-implantitis and other dental diseases with suitable antioxidant supplementation is already one of the objectives of modern toxicological research in the biomaterials field [Citation20, Citation21]. The addition of N-acetyl cysteine in resin-based materials has favored the formulation of a new material in which the intrinsic cytotoxicity of the resin was potentially detoxified [Citation22]. Similarly, the use of Trolox® seems to reduce the cytotoxicity induced by biomaterials [Citation23]. Implants were developed using antioxidant stabilizing compounds such as vitamin E, which was typically added in low concentrations during ethylene polymer consolidation. Clinical data suggest that blended formulations with concentrations of less than 0.1% vitamin E maintain the same physical and mechanical properties and can prevent oxidation for up to 24 months post-implantation [Citation24]. Recently, antioxidant polymers, which are molecules that have natural antioxidants (quercetin and curcumin) incorporated into a polymer backbone, were synthesized to attenuate material-induced oxidative stress [Citation25]. The preparation of silica microspheres through a novel method that uses a polyol-in-oil-in-water (P/O/W) emulsion and sol–gel methods as techniques for stabilizing quercetin has been also reported [Citation26].
In order for new biomaterials with native intrinsic antioxidant properties to be suitable and widely used in the clinical setting, they should provide the benefit of relatively high antioxidant content and continuous local antioxidant potential while the implant is present. In the search for new biocompatible biomaterials that provide antioxidant functionality and do not exacerbate the body’s normal oxidant and inflammatory response, our research group has optimized the synthesis of novel, intrinsically antioxidant, quercetin-based biomaterials, which could be used in dentistry as components in glass ionomer cement and in medicine as replacements for bone implants. Quercetin is a naturally occurring flavonol broadly recognized for its antioxidant and anti-inflammatory properties [Citation27]. We applied the sol–gel technique for this purpose. The sol–gel process is currently one of the most studied and used techniques for the production of high-quality glassy and ceramic materials [Citation28]. Interest in this technique is stimulated by the extreme versatility of the method, which, being highly controllable, has many advantages compared to traditional methods. In recent years, we have extensively adopted sol–gel routes for the formulation of inorganic or hybrid inorganic–organic materials that are potentially useful in the dental and orthopedic fields [Citation29–Citation33].
The addition of quercetin (in weight percentages of 5, 10, or 15%) to an inorganic silica matrix in the synthetic process was aimed at formulating an antioxidant, bioactive, and biocompatible glass material in which the natural molecule firmly integrates into the inorganic network. Liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC-ESI/MS/MS) techniques were applied to study the antioxidant drug release. Ultraviolet–visible (UV–vis) and collision-activated dissociation (CAD) mass spectrometry (MS) techniques were applied to define the integrity of the quercetin skeleton. In vitro bioactivity of synthesized materials, which is indicative of their osseointegration ability, was investigated by soaking the samples in a simulated body fluid (SBF) and using scanning electron microscopy (SEM) to observe the hydroxyapatite formation on the surface. Since one of the recommended and appropriate steps for the biological assessment of medical devices is the in vitro assessment of cytotoxicity of new biomaterials, an MTT assay was carried on the NIH-3T3 murine fibroblast cell line. The ability of the new system to exhibit antioxidant properties was evaluated first by applying DPPH and ABTS methods, and then by a 2,7-dichlorofluorescein diacetate assay on NIH-3T3 mouse fibroblast cells.
2. Materials and methods
2.1. Sol–gel synthesis
Inorganic SiO2 and SiO2/quercetin hybrids (Si/Que) that differed in their drug content (5, 10, and 15%wt) were prepared by means of a sol–gel process (table ).
Table 1. Composition and label of the synthesized systems.
The inorganic silica gel was synthesized by adding tetraethyl orthosilicate (TEOS; Si(OC2H5)4, Sigma-Aldrich) to a solution containing HNO3 (≥65%, Sigma-Aldrich) and distilled water in ethanol 99.8% (Sigma-Aldrich). The acidic environment favors the kinetics of hydrolysis and condensation reactions, and thus affects the microstructural properties of the inorganic matrix [Citation34]. The structural characteristics were also influenced by the H2O/alkoxide molar ratio. When the value was less than four, compact and mesoporous materials were obtained [Citation35]. The molar ratio used for the reagents was TEOS:HNO3:EtOH:H2O=1:1.7:6:2.
To prepare the hybrid materials, quercetin (Sigma-Aldrich) was dissolved in ethanol and added to the synthesized silica sol under stirring. After gelation, the products were air-dried at 50 °C for 24 h to remove the residual solvent.
2.2. Characterization studies
The characterization of the developed materials was implemented using different techniques.
The microstructure of the synthesized gels was studied by SEM (Quanta 200, FEI, Netherlands).
Fourier transform infrared (FTIR) transmittance spectra were recorded in the 400–4000 cm−1 region using a Prestige 21 (Shimadzu, Japan) system, equipped with a deuterated tryglycine sulphate detector with potassium bromide windows, with a resolution of 2 cm−1 (45 scans). KBr pelletized disks containing 2 mg of each sample and 198 mg of KBr were made. The FTIR spectra were processed by Prestige software (IR solution).
The UV–vis spectra of extracts from discs of each different synthesized material, which had previously been soaked in Dulbecco’s Phosphate Buffer Saline (DPBS) SBF, were acquired in the range 200–600 nm using a Shimadzu 1700 spectrophotometer.
Mass spectra were recorded using a Quattro Micro™ triple quadrupole mass spectrometer (Waters/Micromass, Manchester, UK) equipped with an electrospray ionization (ESI) source operating in the positive ion mode. Nitrogen was used as the nebulizer and solvent gas at flow rates of 50 and 500 l/h, respectively. Source and dissolution temperatures were set at 120 °C and 350 °C, respectively. Applied potentials of the electrospray capillary and of the cone were set at 2.50 kV and 10 V, respectively. CAD mass spectra were recorded by introducing argon as a collision gas into the RF-only quadrupole at a pressure of ∼3.0 × 10−3 mbar to minimize multiple collisions. The mass range was recorded from 20 to 800 m z−1, with interchannel and interscan delays of 0.02 s and 0.1 s, respectively. The collision energy used for CAD analysis was 10 eV (Elab). Data acquisition and processing were carried out using the software MassLynx™ version 4.0 supplied with the instrument.
2.3. In vitro bioactivity
The in vivo bone-bonding ability of the synthesized materials was evaluated by an in vitro apatite forming-ability test. Pelletized disks of SiO2 and Si/Que hybrids were soaked for 7, 14, and 21 days in an SBF with an ion concentration nearly equal to that of human blood plasma [Citation36]: Na+ 142.0, K+ 5.0, Ca2+ 2.5, Mg2+ 1.5, Cl− 147.8, HCO3− 4.2, 1.0, SO42− 0.5 mM. Polystyrene bottles containing the samples were placed in a water bath, and during soaking the temperature was kept fixed at 37 °C. Taking into account that the ratio between the total surface of the material exposed to the SBF and its volume influences the reaction of the hydroxyapatite layer formation, a constant ratio of 10 mm2 ml−1 of solution was respected [Citation37]. Moreover, the SBF solution was changed every 2 days to avoid depletion of the ionic species in the SBF due to the formation of biominerals by the samples.
After each soaking period in the SBF, the samples were dried in a desiccator and then subjected to SEM combined with energy-dispersive x-ray spectroscopy (EDS) to evaluate their ability to form an apatite layer on their surfaces.
2.4. In vitro release test
Three discs of each quercetin silica-based material (Si/Que5, Si/Que10, and Si/Que15; 200.0 mg each) were soaked in 7.5 ml DPBS SBF at 37 °C under continuous stirring. LC/UV-ESI/MS analyses were carried out for up to 7 days.
The chromatographic apparatus consisted of an Alliance 2695 separations module equipped with a column heater and a sample chiller and a Waters 2487 dual-wavelength UV detector. Separations were achieved using a Synergy Hydro® C8 reversed-phase column (4.0 μm particle size, 250 × 4.6 mm i.d., Phenomenex) at a flow rate of 0.40 ml min−1 with isocratic elution of 0.1% (v/v) formic acid aqueous solution and acetonitrile (1:3, v/v). The UV detection of quercetin was performed at 380 nm. To generate the calibration curve, several known concentrations of the standard (0–2.0 mM) were injected; the response factors based on the linear regression of a plot of peak area versus concentration were computed. The injection volume was 10 μl. The liquid chromatography (LC) system was coupled to the previously described Quattro Micro™ triple quadrupole mass spectrometer (Waters/Micromass, Manchester, UK).
2.5. Determination of DPPH• scavenging capacity
To estimate the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) scavenging capability, extracts from the investigated matrices (50.0 μl) were added to a DPPH• methanol solution (9.4 × 10−5 M; 1.0 ml final volume) at room temperature. After 30 min, the absorption at 515 nm was measured by a Shimadzu UV-1700 spectrophotometer in reference to a blank. The results were expressed in terms of the percentage decrease of the initial DPPH• radical absorption by the test samples [Citation38].
2.6. Determination of ABTS•+ scavenging capacity
The determination of ABTS•+ solution scavenging capacity was estimated as previously reported [Citation38]. 2,2′-azinobis-(3-ethylbenzothiazolin-6-sulfonic acid (ABTS) radical cation was generated by reacting ABTS (7.0 mM) and potassium persulfate (2.45 mM). The mixture was allowed to stand in the dark at room temperature for 12–16 h. Thus, the ABTS•+ solution was diluted with DPBS (pH 7.4) in order to reach an absorbance of 0.70 at 734 nm. Extracts from investigated matrices (50.0 μL) were added in diluted ABTS•+ solution (1.0 ml final volume). After 6 min of incubation, the absorption at 734 nm was measured by a Shimadzu UV-1700 spectrophotometer in reference to a blank. The results were expressed in terms of the percentage decrease of the initial ABTS•+ absorption by the test samples.
2.7. Cell culture and cytotoxicity assessment
Cytotoxicity was measured through an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2 H-tetrazolium bromide] cell-growth inhibition assay (indirect test) using the NIH-3T3 murine fibroblast cell line. Investigated extracts were obtained from discs of the studied materials, which were previously immersed for 24 h in 3.5 ml of a complete culture medium at 37 °C under continuous stirring.
The cell line, purchased from American Type Culture Collection, was grown in Roswell Park Memorial Institute (RPMI) medium 1640 high-glucose medium supplemented with 10% fetal bovine serum, 50.0 U ml−1 penicillin, and 100.0 μg ml−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
The NIH-3T3 cell line was seeded in 96-multiwell plates at a density of 1.0 × 104 cells/well. After 24 h of incubation, cells were treated with 200 μl culture medium solution containing 50 μl of extracts from silica and silica-quercetin materials (with entrapped quercetin at 5, 10, and 15%wt). At 24, 48, and 72 h of incubation, cells were treated with 150 μl of MTT (0.50 mg ml−1) dissolved in the culture medium for 1 h at 37 °C in a 5% CO2 humidified atmosphere. The MTT solution was then removed, and 100 μl of dimethyl sulfoxide was added to dissolve the originated formazan. Finally, the absorbance of each well at 570 nm was determined using a Tecan SpectraFluor fluorescence and absorbance reader. Cell viability was expressed as percentage of mitochondrial redox activity of the cells treated with the extracts compared to an untreated control [Citation39]. Tests were carried out performing twelve replicate (n = 12) measurements for three samples of each extract (in total: 12 × 3 measurements).
2.8. Measurement of intracellular ROS formation
The levels of intracellular ROS were determined by the change in fluorescence resulting from the oxidation of the fluorescent probe, 2′,7′-dichlorofluorescein diacetate (DCFH-DA). When applied to intact cells, DCFH-DA readily diffuses through the cell membrane and is hydrolyzed enzymatically by intracellular esterases to nonfluorescent DCFH. In the presence of ROS, DCFH is oxidized to highly fluorescent DCF, whose fluorescent intensity is proportional to the amount of ROS formed intracellularly [Citation40].
The NIH-3T3 cell line, seeded in 96-multiwell plates at a density of 1.0 × 104 cells/well, was incubated with DCFH-DA (10 μM) in DPBS for 30 min. At the end of incubation, the DCFH-DA solution was removed and cells were cotreated with Que15 or Si/Que15 materials (50 μl) and H2O2 (400.0 μM). The fluorescence intensity was measured at 485 nm excitation and 535 nm wavelength in a Tecan SpectraFluor fluorescence and absorbance reader. Results were expressed as percentages relative to oxidized cell lines arbitrarily set at 100%.
3. Results and discussion
3.1. Characterization of synthesized materials
All the synthesized materials appeared transparent, glassy, and reddish in color. They were characterized by the same morphology. Their compact structure could be due to the H2O/alkoxide molar ratio used. In fact, it is known that when the H2O/alkoxide molar ratio has a value less than 4, compact and mesoporous materials are obtained [Citation35].
Figure shows SEM micrographs of the Si/Que systems. No appreciable difference in surface morphology could be observed. The organic and inorganic phases were indistinguishable, thus confirming the hybrid feature of the synthesized sol–gel materials.
Information on the structural organization of the bioactive molecule and silica matrix within the synthesized materials was determined by FTIR spectroscopy.
The FTIR spectrum for pure quercetin is shown in figure (a), where its characteristic bands [Citation41] were detected. OH groups stretching were detectable at 3406 and 3283 cm−1, whereas OH bending of the phenol function was detectable at 1379 cm−1. The C=O aryl ketonic stretch absorption was evident at 1666 cm−1. C=C aromatic ring stretch bands were detectable at 1610, 1560, and 1510 cm−1. The in-plane bending band of C–H in aromatic hydrocarbon was detectable at 1317 cm−1, and out-of-plane bending bands were evident at 933, 820, 679, and 600 cm−1. Bands at 1263, 1200, and 1165 cm−1 were attributable to the C–O stretching in the aryl ether ring, the C–O stretching in phenol, and the C–CO–C stretch and bending in ketone, respectively.
Figure 2. FTIR spectra of (a) pure quercetin, (b) Si/Que hybrid systems. Spectra are vertically shifted for clarity.
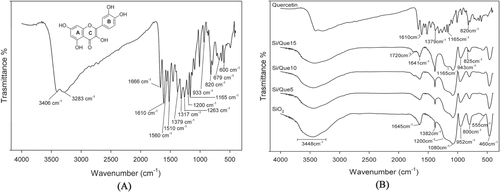
To identify the interaction between the quercetin and the SiO2 matrix, the FTIR spectra of pure quercetin (Que) and quercetin-free silica (SiO2) were compared to those of the quercetin-entrapped silica based-materials (Si/Que), as shown in figure (b).
In the SiO2 spectrum (figure (b)), all typical peaks of the silica sol–gel materials [Citation42–Citation44] were visible. The strong band at 1080 cm−1 with a shoulder at 1200 cm−1, and the peaks at 800 cm−1 and 460 cm−1, were ascribed to asymmetric and symmetric Si-O stretching motions and the bending Si-O-Si mode, respectively. The band at 952 cm−1 was assigned to Si-OH bond vibrations [Citation45] and the low-intensity band at 555 cm−1 was attributed to residual four-membered siloxane rings in the silica network [Citation42, Citation46–Citation49]. Moreover, the 1382 cm−1 sharp peak was due to N–O stretching of residual nitrate anions resulting from HNO3 used as catalyst in the synthesis procedure; the broad, intense band at 3448 cm−1 and the peak at 1645 cm−1 were due to OH stretching and bending vibrations in the hydration water. The position and the shape of the latter bands suggest the presence of hydrogen-bonded solvent molecules (H2O) and hydrogen-bonded OH groups attached to the Si atoms [Citation50].
All discussed peaks were also visible in the Si/Que materials spectra (figure (b)). However, the drug addition caused a slight decrease in their intensity and the down-shift of the bands of Si-OH, which appeared at 943 cm−1. These changes could be due to interactions between the inorganic matrix and the quercetin, which caused variations in bond length, and thus of bond strength, in the SiO2 network. In particular, the down-shift of the Si–OH signal, together with the broadening of the OH band at 3448 cm−1, suggested the formation of H-bonds, which involved the hydroxyl groups of the silica matrix. These findings were confirmed by the observation that in these spectra, some typical peaks of quercetin were also detectable in a flavonol concentration-dependent manner. In particular, OH bending of the phenols band at 1379 cm−1 appeared broadened and had a higher intensity in the spectra of the hybrid materials, so that it overlapped with the nitrate peak. The C–CO–C stretch and bending in the ketone band also appeared to be slightly broadened at 1165 cm−1. The slight shift, the broadening, and the increase in terms of energy absorption of the OH bending band and the slight broadening of the ketone band suggested that quercetin was associated with silica matrix OH groups through hydrogen bonds that involved the OH of phenols and the C=O of the C ring. Changes were detected in the region of the double bond-related absorptions, where the bands at 1720 and 1641 cm−1 were evident. The weak intensity of the first band (absent in both the quercetin and the SiO2 spectrum) allowed us to hypothesize that structural modification on the flavonol C-ring was a consequence of the oxidation of the γ-pyrone ring in the molecule (ring C). The conjunction loss among the aromatic B-ring and the C-ring consisted of the up-shifts of the C=C aromatic ring stretching band at 1610 cm−1 at a higher absorption value (1641 cm−1). Aromatic out-of-plane C-H bending vibrations at 820 and 793 cm−1 were also up-shifted at 825 and 797 cm−1, respectively.
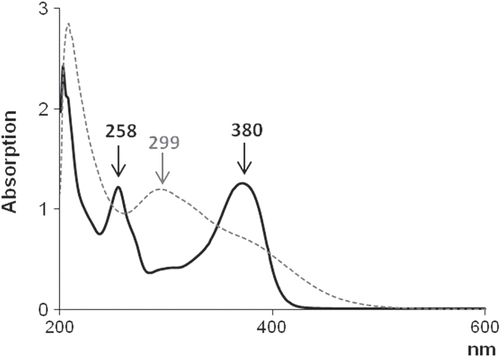
To confirm the structural modification of the quercetin skeleton, UV–vis spectroscopic analysis was equally performed on pure quercetin dissolved in DPBS and extracts from the discs of quercetin-entrapped silica materials previously soaked in DPBS for 1 h (figure ). Quercetin exhibited two absorption bands at 258 and 380 nm. The first one was considered to be associated with absorption due to the B-ring cinnamoyl system, and the second with absorption involving the A-ring benzoyl system [Citation51]. Upon sol–gel synthesis, a new absorption band appeared around 299 nm; the absorption band at 380 nm became less pronounced, and at 258 nm it disappeared. This absorption spectrum was in agreement with the reported absorption spectrum of 2-(3′,4′-dihydroxybenzoyl)-2,4,6-trihydroxybenzofuran-3(2H)-one [Citation51], a quercetin-oxidized derivative commonly produced in aqueous solution and oxygen-free conditions. This finding allowed us to hypothesize that the oxidation process was achieved when sol–gel synthesis was performed and promoted by acidic condition and ethanol presence.
Figure 3. UV–vis spectra of quercetin (solid black curve) and extracts from quercetin-entrapped silica materials (dashed gray curve).
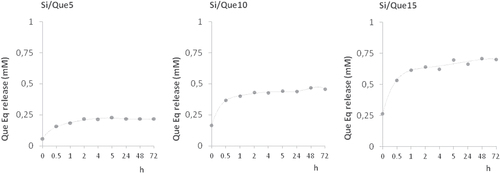
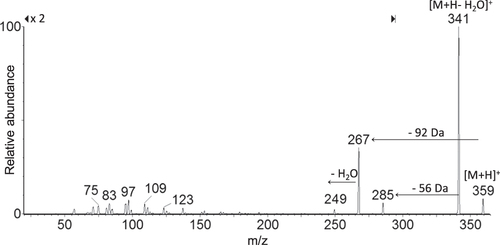
When the potential release of the flavonol was addressed applying LC-ESI/MS/MS analyses on extracts obtained from discs of each investigated material, a slow and steady release of one single product with a retention time 0.5 min less than pure quercetin was recorded. The structural identity of the quercetin derivative was elucidated through the MS technique. The MS/MS spectrum of the [M + H]+ ion at m/z 359 provided fragment ions at m/z 341 [M + H-H2O]+, 285, 267, and 249 (figure ). The proposed synthesis pathway and mass fragmentation pattern of the tentatively identified molecule are shown in figures (a) and (b), respectively. The slow and steady release observed could be due to the high silanol content and the hydrophilic surface of the silica inorganic network, which on one side was able to increase drug loading through the hydrogen bonds’ onset, and on the other allowed drug release to slow down. The amount of drug released increased proportionally to the initial quercetin loaded. Matrix dissolution in DPBS and diffusion could play a key role in the observed release rate (figure ).
3.2. Bioactivity test
All samples were observed by SEM after 7, 14, and 21 days of soaking in SBF (figure ). The formation of crystals with the typical globular shape of the hydroxyl-apatite was detected on the surface of all samples after 7 days of immersion in SBF. EDS analysis showed that the ratio between the atomic content of Ca and P of the observed globules was also 1.6 in hydroxyl-apatite [Citation37]. The amount of those crystals increases with the time of exposure to the SBF, and they entirely cover the surface of all disks after 21 days of incubation in the solution. Any significant difference in terms of precipitate apatite amount is recorded as a function of quercetin content. Therefore, the quercetin content does not affect the bioactivity of the samples.
Figure 7. SEM micrographs of Si/Que5, Si/Que10, and Si/Que15 systems after 7 days and 21 days of soaking in SBF. EDS analysis of the globule in the box.
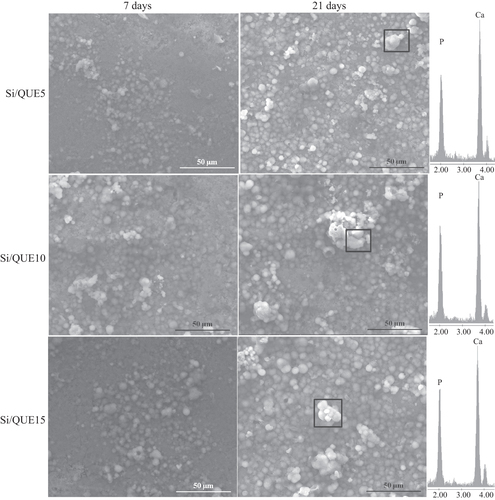
The formation of apatite after soaking in SBF can be explained by the presence of Si-OH groups on their surfaces [Citation29]. These groups, combined with the Ca2+ ions present in the fluid, originate the increase of positive charges on their surfaces. The Ca2+ ions combine with the negative charge of the phosphate ions to form amorphous phosphate, which spontaneously transforms into hydroxyl-apatite [Ca10(PO4)6(OH)2] [Citation52]. The obtained results suggest that quercetin is released in SBF, leaving the Si-OH free to interact, and it does not influence the electrostatic interactions involved in the nucleation process or the ion exchanges.
3.3. Antiradical properties of synthesized materials
To investigate the radical scavenging capacity of the synthesized materials, DPPH and ABTS tests were performed. Both methods are based on the use of a radical species as probe. The activity was compared to that of pure quercetin (figure ). Although all the investigated samples massively reduced the radical species target, it was noted that for the pure molecules, the scavenging efficiency of the new materials was strongly dependent on the quercetin concentration therein.
Figure 8. Radical scavenging capacity (RSC, %) of Si/Que5, Si/Que10, and Si/Que15, and silica-free quercetin samples (Que5, Que10, and Que15) towards DPPH• radical, ABTS•+. Values, reported as percentage versus a blank, are the mean ±SD of measurements carried out on three samples (n = 3) analyzed three times.
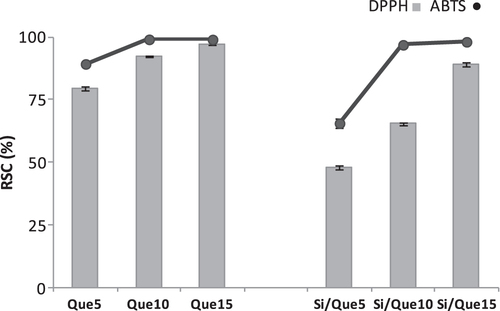
Indeed, initial quercetin concentration seemed to play a predominant role in the antiradical capability performance. When Si/Que10 and Si/Que15 samples were tested, they were able to reduce the radical species, just as pure quercetin did. The presence of a lower dose of quercetin defined a decrease in the antioxidant power. This finding suggested that the preservation of the antioxidant properties of quercetin could be realized by adopting appropriate ratios between the concentration levels of the inorganic and organic materials. The weak antiradical capability of Si/Que5, as opposed to the Que5 scavenging efficacy, highlighted the synthesis of materials in which the flavonol underwent structural modifications. It is well known that the presence of the 2,3-double bond, the 3-hydroxyl group, and the o-catechol group (3′,4′-OH) are determining structural features for the high antioxidant capacity of quercetin [Citation53]. The o-catechol function retention in the B-ring could explain the antioxidant data obtained.
3.4. Influence of SiO2/quercetin-synthesized materials on mitochondria and cell proliferation
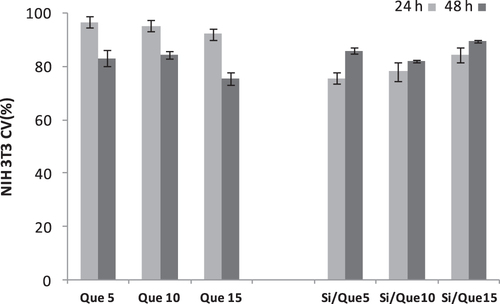
To assess the influence of the extracts from the synthesized sol–gel materials on mitochondria and cell proliferation, an MTT cytotoxicity assay was performed on the NIH-3T3 murine fibroblast cell line. The cell line was treated for exposure times of 24 and 48 h with extracts obtained by placing discs of the investigated materials in a complete culture medium for 24 h.
The quantitative measurement of the extracellular reduction of the yellow-colored water-soluble tetrazolium dye to insoluble formazan crystals allowed us to state that extracts obtained by the different samples affect cell viability and the proliferation of tested cells (figure ). It was observed that all the tested samples were able to cause only mild in vitro suppression of cell functions to levels that would be acceptable on the basis of standards used to evaluate alloys and composites (<25% suppression of deydrogenases activity). In particular, synthesized materials showed an improved time-dependent biocompatibility. This effect was not recorded for pure quercetin, which was able to exert an antiproliferative effect at 48 h that was stronger than at 24 h.
Figure 9. Cell viability (CV, %) toward NIH-3T3 cells of Si/Que5, Si/Que10, and Si/Que15, and silica-free quercetin samples (Que5, Que10, and Que15) at 24 h and 48 h exposure times by means of MTT test results. Values, reported as percentage versus an untreated control, are the mean ±SD of measurements carried out on three samples (n = 3) analyzed 12 times.
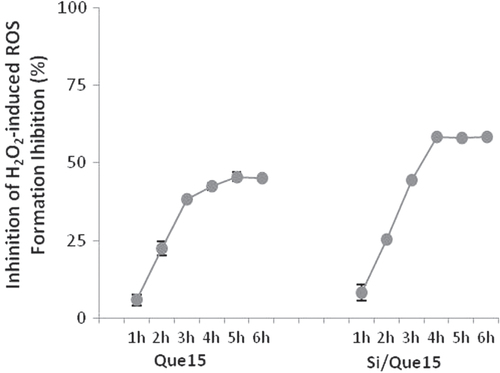
3.5. Effect of Si/Que15 system on intracellular ROS generation
To examine whether the extracts from Si/Que15 synthesized materials could inhibit and/or prevent ROS generation induced by the known free radical generator hydrogen peroxide, the NIH-3T3 cell line was incubated with its extract and H2O2 (400 μM). The assay was performed for 6 h and the DCF fluorescence formation was determined by taking data every hour. The addition of the Si/Que15 extract sample massively reduced the percentage of ROS formation in a time exposure-dependent manner. After 3 h, an inhibition of ROS formation equal to 64.0% was recorded. The antioxidant potential of the Si/Que15 extract appeared to be greater than that exerted by Que15 (figure ), probably because of its higher bioavailability and permeability. The accessibility to lipophilic cellular membranes could explain the decrease of H2O2-induced intracellular ROS production. H2O2 is likely to amplify intracellular ROS production by generating ROS in the medium and/or cellular membranes, as well as in the intracellular matrix. Therefore, differences in the distribution in the medium/cellular membranes between quercetin and its derivative may greatly affect their effectiveness for H2O2-induced intracellular ROS production when added concurrently with H2O2 to cultured cells. Furthermore, quercetin instability and its metabolic conversion explain the reason for the lack of a significant effect of the quercetin aglycon in the pretreatment with the 3T3 mouse fibroblast cell system [Citation54].
4. Conclusions
Quercetin, a member of the flavonoids family and one of the most prominent dietary antioxidants, is claimed to exert beneficial health effects. These include protection against various conditions such as osteoporosis, certain forms of cancer, pulmonary and cardiovascular diseases, and premature aging [Citation55]. It is suggested that quercetin’s ability to scavenge highly reactive oxygen and nitrogen species, such as peroxynitrite and the hydroxyl radical, is involved in these possible beneficial health effects. To counteract peri-implant disease, which can be caused by ROS overproduction, the synthesis of an implant functionalized with the flavonol was achieved. Chemical characterization allowed us to state that the acidic condition of the sol–gel synthesis addressed the preparation of hybrid materials in which C-ring structural features of quercetin were modified. The quercetin derivative retained the o-catecholic function determinant for antioxidant capability.
Data obtained for this new hybrid material suggest that it can combine the features required for a high-quality biomaterial and important antioxidant effectiveness, causing an in vitro suppression of dehydrogenases activity <25%, which is closely related to only mild cell function suppression. On the basis of these considerations, this work forms the basis of a new research field regarding components of glass ionomer cement with intrinsic antioxidant properties as an alternative effective strategy for preventing the onset of peri-implant diseases.
Acknowledgment
ESI/MS spectra were obtained using a LC-TQ MS instrument of the CRdC ‘Analisi e monitoraggio del rischio ambientale’, AMRA (P.O.R. 2000–2006, misura 3.16).
References
- OshidaYTunaE BAktorenOGencayK 2010 Dental implant systems Int. J. Mol. Sci. 11 1580 1678 1580–678 10.3390/ijms11041580
- LeeuwenburghS C G 2008 Trends in biomaterials research: an analysis of the scientific programme of the World Biomaterials Congress 2008 Biomaterials 29 3047 3052 3047–52 10.1016/j.biomaterials.2008.04.032
- ArcosDIzquierdo-BarbaIVallet-RegiM 2009 Promising trends of bioceramics in the biomaterials field J. Mater. Sci. Mater. Med. 20 447 455 447–55 10.1007/s10856-008-3616-x
- RatnerB DBryantS J 2004 Biomaterials: where we have been and where we are going Ann. Rev. Biomed. Eng. 6 41 75 41–75 10.1146/annurev.bioeng.6.040803.140027
- BoccacciniA RBlakerJ J 2005 Bioactive composite materials for tissue engineering scaffolds Expert Rev. Med. Devices. 2 303 317 303–17 10.1586/17434440.2.3.303
- PoliniABaiHTomsiaA P 2013 Dental applications of nanostructured bioactive glass and its composites Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 5 399 410 399–410 10.1002/wnan.1224
- ArcosDVallet-RegiM 2010 Sol-gel silica-based biomaterials and bone tissue regeneration Acta Biomater. 6 2874 2888 2874–88 10.1016/j.actbio.2010.02.012
- NavarroMMichiardiACastanoOPlanellJ A 2008 Biomaterials in orthopaedics J. R. Soc. Interface 5 1137 1158 1137–58 10.1098/rsif.2008.0151
- KokuboTKimH-MKawashitaM 2003 Novel bioactive materials with different mechanical properties Biomaterials 24 2161 2175 2161–75 10.1016/S0142-9612(03)00044-9
- BrunnerT J 2006 In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility Environ. Sci. Technol. 40 4374 4381 4374–81 10.1021/es052069i
- ChangJ-SChangK L BHwangD-FKongZ-L 2007 In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line Environ. Sci. Technol. 41 2064 2068 2064–8 10.1021/es062347t
- RabolliV 2010 Influence of size, surface area and microporosity on the in vitro cytotoxic activity of amorphous silica nanoparticles in different cell types Nanotoxicology 4 307 318 307–18 10.3109/17435390.2010.482749
- YangHLiuCYangDZhangHXiZ 2009 Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition J. Appl. Toxicol. 29 69 78 69–78 10.1002/jat.1385
- Velasco-OrtegaEJosACameanA MPato-MoureloJSegura-EgeaJ J 2010 In vitro evaluation of cytotoxicity and genotoxicity of a commercial titanium alloy for dental implantology Mutat. Res. 702 17 23 17–23 10.1016/j.mrgentox.2010.06.013
- PietropaoliDOrtuESeverinoMCiarrocchiIGattoRMonacoA 2013 Glycation and oxidative stress in the failure of dental implants: a case series BMC Res. Notes 6 296 10.1186/1756-0500-6-296
- SooryM 2008 A role for non-antimicrobial actions of tetracyclines in combating oxidative stress in periodontal and metabolic diseases: a literature review Open Dent. J. 2 5 12 5–12 10.2174/1874210600802010005
- KaroussisI KMullerSSalviG EHeitz-MayfieldL J ABraggerULangN P 2004 Association between periodontal and peri-implant conditions: a 10-year prospective study Clin. Oral Implants Res. 15 1 7 1–7 10.1111/j.1600-0501.2004.00982.x
- CarnelioSKhanS ARodriguesG 2008 Definite, probable or dubious: antioxidants trilogy in clinical dentistry Br. Dent. J. 204 29 32 29–32 10.1038/bdj.2007.1186
- LiskmannSVihalemmTSalumOZilmerKFischerKZilmerM 2007 Characterization of the antioxidant profile of human saliva in peri-implant health and disease Clin. Oral Implants Res. 18 27 33 27–33 10.1111/j.1600-0501.2006.01296.x
- SooryM 2009 Redox status in periodontal and systemic inflammatory conditions including associated neoplasias: antioxidants as adjunctive therapy? Infect Disord.: Drug Targets 9 415 427 415–27
- GalliCPasseriGMacalusoG M 2011 FoxOs, Wnts and oxidative stress-induced bone loss: new players in the periodontitis arena J. Periodontal Res. 46 397 406 397–406 10.1111/j.1600-0765.2011.01354.x
- AttWYamadaMKojimaNOgawaT 2009 N-Acetyl cysteine prevents suppression of oral fibroblast function on poly(methylmethacrylate) resin Acta Biomater. 5 391 398 391–8 10.1016/j.actbio.2008.07.021
- SoheiliM EGoldbergMStanislawskiL 2003 In vitro effects of ascorbate and Trolox on the biocompatibility of dental restorative materials Biomaterials 24 3 9 3–9 10.1016/S0142-9612(02)00221-1
- CostaLCarpentieriIBraccoP 2009 Post electron-beam irradiation oxidation of orthopaedic Ultra-High Molecular Weight Polyethylene (UHMWPE) stabilized with vitamin E Polym. Degrad. Stab. 94 1542 1547 1542–7 10.1016/j.polymdegradstab.2009.04.023
- WattamwarP P 2012 Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants Acta Biomater. 8 2529 2537 2529–37 10.1016/j.actbio.2012.03.022
- HoK Y 2014 Preparation and characterization of quercetin-loaded silica microspheres stabilized by combined multiple emulsion and sol-gel processes Chem. Ind. Chem. Eng. Q 10.2298/CICEQ131002010K
- RatheePChaudharyHRatheeSRatheeDKumarVKohliK 2009 Mechanism of action of flavonoids as anti-inflammatory agents: a review Inflammation Allergy: Drug Targets 8 229 235 229–35 10.2174/187152809788681029
- CatauroMBollinoFPapaleF 2014 Biocompatibility improvement of titanium implants by coating with hybrid materials synthesized by sol-gel technique J. Biomed. Mater. Res. A 102 4473 4479 4473–9 10.1002/jbm.a.35116
- CatauroMPapaleFBollinoFGallicchioMPacificoS 2014 Biological evaluation of zirconia/PEG hybrid materials synthesized via sol-gel technique Mater. Sci. Eng. C 40 253 259 253–9 10.1016/j.msec.2014.04.001
- CatauroMBollinoFPapaleFGallicchioMPacificoS 2014 Synthesis and chemical characterization of new silica polyethylene glycol hybrid nanocomposite materials for controlled drug delivery J. Drug Delivery Sci. Technol. 24 320 325 320–5 10.1016/S1773-2247(14)50069-X
- CatauroMBollinoFVeronesiPLamannaG 2014 Influence of PCL on mechanical properties and bioactivity of ZrO2-based hybrid coatings synthesized by sol–gel dip coating technique Mater. Sci. Eng. C 39 344 351 344–51 10.1016/j.msec.2014.03.025
- CatauroMBollinoFPapaleFGallicchioMPacificoS 2015 Influence of the polymer amount on bioactivity and biocompatibility of SiO2/PEG hybrid materials synthesized by sol-gel technique Mater. Sci. Eng. C 48 548 555 548–55 10.1016/j.msec.2014.12.035
- CatauroMBollinoFPapaleFMarcianoSPacificoS 2015 TiO2/PCL hybrid materials synthesized via sol-gel technique for biomedical applications Mater. Sci. Eng. C 47 135 141 135–41 10.1016/j.msec.2014.11.040
- CurranM DStiegmanA E 1999 Morphology and pore structure of silica xerogels made at low pH J. Non-Cryst. Solids 249 62 68 62–8 10.1016/S0022-3093(99)00237-9
- RoJ CChungI J 1991 Structures and properties of silica gels prepared by the sol-gel method J. Non-Cryst. Solids 130 8 17 8–17 10.1016/0022-3093(91)90151-U
- KokuboTKushitaniHSakkaSKitsugiTYamamuroT 1990 Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W J. Biomed. Mater. Res. 24 721 734 721–34 10.1002/jbm.820240607
- KokuboTTakadamaH 2006 How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27 2907 2915 2907–15 10.1016/j.biomaterials.2006.01.017
- PacificoS 2014 Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region Food. Res. Int. 64 188 199 188–99 10.1016/j.foodres.2014.06.020
- PacificoS 2013 Apolar Laurus nobilis leaf extracts induce cytotoxicity and apoptosis towards three nervous system cell lines Food Chem. Toxicol. 62 628 637 628–37 10.1016/j.fct.2013.09.029
- PacificoS 2014 Neuroprotective potential of Laurus nobilis antioxidant polyphenol-enriched leaf extracts Chem. Res. Toxicol. 27 611 626 611–26 10.1021/tx5000415
- HeneczkowskiMKopaczMNowakDKuzniarA 2001 Infrared spectrum analysis of some flavonoids Acta Pol. Pharm. 58 415 420 415–20
- AdeogunM JHayJ N 2001 Structure control in sol-gel silica synthesis using ionene polymers. 2: evidence from spectroscopic analysis J. Sol-Gel Sci. Technol. 20 119 128 119–28 10.1023/A:1008795321600
- InnocenziP 2003 Infrared spectroscopy of sol-gel derived silica-based films: a spectra-microstructure overview J. Non-Cryst. Solids 316 309 319 309–19 10.1016/S0022-3093(02)01637-X
- PiccirilloA MBorysenkoS SBorysenkoS D 2011 Qualitative analysis behaviour of the solutions of impulsive differential systems AAPP Atti della Accademia Peloritana dei Pericolanti, Classe di Scienze Fisiche, Matematiche e Naturali 89 C1A8902002
- SimonVEniuDGritcoASimonS 2007 Thermal and spectroscopic investigation of sol-gel derived aluminosilicate bioglass matrices J. Optoelectron. Adv. Mater. 9 3368 3371 3368–71
- NedelecJ MHenchL L 1999 Ab initio molecular orbital calculations on silica rings J. Non-Cryst. Solids 255 163 170 163–70 10.1016/S0022-3093(99)00367-1
- YoshinoHKamiyaKNasuH 1990 IR study on the structural evolution of sol-gel-derived silica gels in the early stage of conversion to glasses J. Non-Cryst. Solids 126 68 78 68–78 10.1016/0022-3093(90)91024-L
- ChenY-CLiuC-PYangC-KHuangB-YLiuC-Y 2013 Preparation and release properties of sol-gel encapsulated proteins J. Anal. Sci, Methods Instrum. 3 6 10.4236/jasmi.2013.33A002
- PiccirilloA MCiarlettaMBorysenkoS D 2012 Impulsive wendroff's type inequalities and their applications AAPP Atti della Accademia Peloritana dei Pericolanti, Classe di Scienze Fisiche, Matematiche e Naturali 90 A2
- GeorgievaIDanchovaNGutzovSTrendafilovaN 2012 DFT modeling, UV–vis and IR spectroscopic study of acetylacetone-modified zirconia sol-gel materials J. Mol. Model 18 2409 2422 2409–22 10.1007/s00894-011-1257-3
- RamesovaSSokolovaRDeganoIBulickovaJZabkaJGalM 2012 On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions Anal. Bioanal. Chem. 402 975 982 975–82 10.1007/s00216-011-5504-3
- OhtsukiCKokuboTYamamuroT 1992 Mechanism of apatite formation on CaOSiO2P2O5 glasses in a simulated body fluid J. Non-Cryst. Solids 143 84 92 84–92 10.1016/S0022-3093(05)80556-3
- SilvaM MSantosM RCarocoGRochaRJustinoGMiraL 2002 Structure-antioxidant activity relationships of flavonoids: a re-examination Free Radical Res. 36 1219 1227 1219–27 10.1080/198-1071576021000016472
- ShiraiMYamanishiRMoonJ-HMurotaKTeraoJ 2002 Effect of quercetin and its conjugated metabolite on the hydrogen peroxide-induced intracellular production of reactive oxygen species in mouse fibroblasts Biosci. Biotechnol. Biochem. 66 1015 1021 1015–21 10.1271/bbb.66.1015
- BootsA WHaenenG R M MBastA 2008 Health effects of quercetin: from antioxidant to nutraceutical Eur. J. Pharmacol. 585 325 337 325–37 10.1016/j.ejphar.2008.03.008


![Figure 5. (a) Proposed synthesis pathway of the tentatively identified quercetin derivative. (b) Proposed fragmentation pattern of the tentatively identified quercetin derivative ([M + H]+ at m/z 359).](/cms/asset/436bce70-7741-4e87-81df-7ffa3311323d/tsta_a_11661297_f0005_ob.jpg)