Abstract
Objective
The prevalence of dementia and cognitive impairment is higher in Aboriginal Australians compared to the national population, increasing the need to understand cognitive impairment in this at‐risk population. This article reports normative data for a range of commonly used cognitive tests, in a population‐based small normative sample of older Aboriginal Australians living in urban/regional New South Wales.
Method
Participants included a representative random sample of community‐dwelling older adults (60-years and older) with no cognitive impairment (n = 31), mild cognitive impairment (MCI; n = 38), or dementia diagnosis (n = 35), all from the Koori Growing Old Well Study. Cognitive tests included the Addenbrooke's Cognitive Examination Revised (ACE‐R), Digit Span (Forward and Backward), Logical Memory, and the Oral Trail Making Test (A and B).
Results
Descriptive statistics and percentile scores for each test were reported for the normative sample. Comparison of performance between the diagnostic groups showed significant differences between the groups on most cognitive tests. The control group consistently performed better than the dementia group; and better than the MCI group on all tests, except for simple attention and sequencing tasks (Digit Span Forward and Oral Trail Making A). The MCI group also scored better than the dementia group on all tests, except for the Logical Memory Recognition task.
Conclusion
Results support the utility of these cognitive tests that are commonly used in clinical and research settings, and demonstrate that these tests can discriminate between diagnostic groups in Aboriginal Australians. The normative data provided will enhance cognitive assessment of individuals within this population.
Funding information National Health and Medical Research Council, Grant/Award Number: 1105106510347; NHMRC‐ARC Dementia Research Development Fellowship, Grant/Award Number: 1103312; Serpentine Foundation Fellowship
WHAT IS ALREADY KNOWN ABOUT THIS TOPIC?
There is a limited evidence‐base for assessing cognitive function in Aboriginal and Torres Strait Islander populations.
Only certain general cognitive screening measures have been validated in Aboriginal and Torres Strait Islander cohorts, but more sensitive and diverse measures are also needed.
Given the increased rates of dementia and cognitive decline in this population, there is a need for appropriate cognitive test norms to guide assessment, diagnosis, and care decisions.
WHAT THIS TOPIC ADDS?
This study provides normative data for a selection of commonly used neuropsychological tests in older Aboriginal and Torres Strait Islander Australians.
These tests are sensitive to mild cognitive impairment and dementia in this population.
This normative data will enhance cognitive assessment for many older Aboriginal and Torres Strait Islander peoples, particularly in urban and regional settings.
INTRODUCTION
Dementia is becoming an increasing concern in today's society. In Australia alone, the number of people with dementia is expected to triple by the year 2050, reaching approximately 900,000 compared to 298,000 in 2011 (Australian Institute of Health and Welfare, Citation2012). The prevalence of dementia in Aboriginal and Torres Strait Islander Australians (respectfully referred to hereon as Aboriginal) is higher than in non‐Aboriginal Australians (Li et al., Citation2014; Smith et al., Citation2008; Zann, Citation1994). In urban and rural communities, recent evidence has shown that the age‐standardised prevalence of dementia in older Aboriginal Australians is three times higher (at 21.0%) than all Australians aged 60-years or older (at 6.8%) (Australian Institute of Health and Welfare, Citation2012; Radford et al., Citation2015a). Given that the number and proportion of older Aboriginal Australians is also increasing rapidly (ABS, Citation2014), it is vital that we understand cognitive impairment and dementia in this population.
Many commonly used cognitive assessment tools are heavily influenced by Western cultures (Davidson, Citation1995), and hence the validity of these cognitive tests in Indigenous populations is unclear. In light of this, the Kimberley Indigenous Cognitive Assessment (KICA‐Cog) (LoGiudice et al., Citation2006; Smith et al., Citation2009) was developed for remote Aboriginal populations and is increasingly used widely, with short‐form (LoGiudice et al., Citation2010) and urban adaptations (Radford et al., Citation2015b). This tool has greatly enhanced the cognitive assessment of older Aboriginal people, but the KICA‐Cog is insufficient as it may be less sensitive to early cognitive decline (Radford et al., Citation2015b); and, like the ubiquitous Mini‐Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, Citation1975), it lacks a robust assessment of executive functions.
Selected cognitive tests that have been well validated in other populations might also be appropriate for the assessment of Aboriginal people; however, there is a striking lack of normative data, particularly in urban and regional Aboriginal communities. Radford et al. (Citation2015b) validated three cognitive screening tools in an urban/regional Aboriginal Australian sample (MMSE, modified KICA, and Rowland Universal Dementia Assessment Scale [RUDAS]); however, there is no data on more comprehensive assessments or those assessing specific cognitive domains. In addition, Aboriginal Australians have been under‐represented in large‐scale population studies of ageing and cognitive decline (Anstey et al., Citation2011). Given that normative data from the relevant population are needed to interpret individual test results fairly and reliably (Dingwall & Cairney, Citation2010), it is important that appropriate norms are developed within Aboriginal populations. Such normative data will add to the clinical applicability and interpretation of specific cognitive tests in Aboriginal people (Dingwall, Lindeman, & Cairney, Citation2014). The availability of appropriate cognitive test norms in this population could facilitate the inclusion of older Aboriginal people in major dementia prevention and treatment trials that are more representative of the Australian population as a whole.
Therefore, the primary aim of this article is to provide normative data on several cognitive tests, using a small but representative, population‐based sample of cognitively intact older Aboriginal Australians from the Koori Growing Old Well Study (KGOWS) (Radford et al., Citation2014). As a secondary aim, this article also characterises performance across the cognitive spectrum, by comparing healthy control participants to those with a diagnosis of mild cognitive impairment (MCI), or dementia. It was expected that control participants would perform better on all tests compared to those with MCI or dementia; and that those with MCI would perform better than individuals with dementia.
METHOD
Procedure
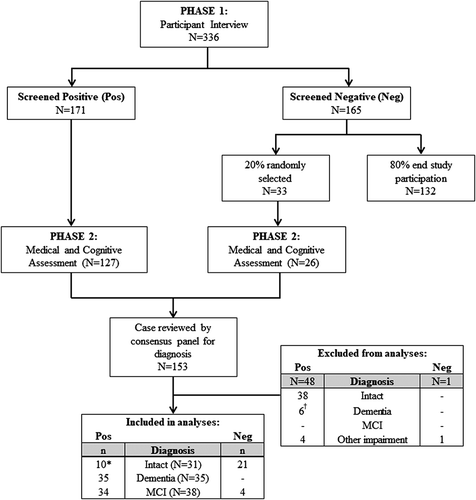
Extended details of the KGOWS protocol and methods are published elsewhere (Radford et al., Citation2014, Citation2015a). Briefly, a cross‐sectional population‐based study was undertaken with five Aboriginal communities across New South Wales, Australia (two metropolitan/urban and three regional sites). Each community had a local Aboriginal Community Controlled Health Organisation and/or a mainstream Aboriginal Health Manager, Elders Groups, Local Aboriginal Land Council, and other community organisations with which the research team had established working relationships in research and/or health service delivery (see Radford et al., Citation2014). Individuals who consented to take part in the study undertook a structured interview reviewing their life history, general health and well‐being, and dementia screening (phase 1). Dementia screening tests included the MMSE (Folstein et al., Citation1975); the modified Kimberley Indigenous Cognitive Assessment (mKICA) (Radford & Mack, Citation2012); and the RUDAS (Storey, Rowland, Basic, Conforti, & Dickson, Citation2004). Participants who met predefined screening cut‐offs (with high sensitivity) of ≤26 on the MMSE, ≤35 on the mKICA, and/or ≤ 25 on the RUDAS proceeded to the study phase 2, as did a 20% random sample of individuals who scored above all three screening cut‐offs. Phase 2 assessment included a 90 min medical and cognitive assessment; and a contact person (relative or friend) interview.
The study was approved by the Aboriginal Health and Medical Research Council (AHMRC; 615/07), the University of New South Wales Human Research Ethics Committee (HREC 08003), and NSW Population & Health Services Research Ethics Committee (AU RED Ref: HREC/09/CIPHS/65; Cancer Institute NSW Ref: 2009/10/187).
Participants
Data collection was carried out in two phases (see Figure 1). All Aboriginal and Torres Strait Islander people aged 60-years and older, living in the five study catchment areas for at least 6 months, were eligible to participate; and 62% (n = 336) of the total population consented to and completed the phase 1 interview (approximately 2 hr). Individuals who screened positive for possible cognitive impairment or dementia on at least one of the screening tools were asked to complete the phase 2 medical assessment (approximately 90-min; n = 171). Of the participants who screened negative at phase 1 interview (n = 165), 20% were randomly selected for phase 2 (n = 33); with the majority consenting to the medical assessment (n = 26). Only 20% were selected for the medical as it was important to validate the cognitive screening tools (the panel was blind to screening result), but it was not feasible to include all participants in the phase 2 assessments (Radford et al., Citation2015b). In total, 153 participants took part in the phase 2 medical assessment. See Radford et al. (Citation2015a) for a full description of inclusion/exclusion criteria and reasons for non‐participation at each phase. Data were then reviewed by a clinical consensus panel (comprising three or more clinicians, with at least one geriatrician and one clinical neuropsychologist) for diagnosis of dementia, using internationally recommended criteria for “all‐cause” dementia (McKhann et al., Citation2011) or MCI (Winblad et al., Citation2004). The medical and consensus process took into account general health and medical history, neurological examination, cognitive assessment, and an extensive interview with the contact person, which asked about the general health, behaviour, everyday cognition, and activities of daily living of the participant. All participants were English‐speaking. Participants provided written informed consent or, when unable to provide written consent, gave verbal assent with written consent obtained from the appropriate relative/caregiver.
We aimed to ensure that 20% of the total study population with no cognitive impairment were included in the normative sample. Therefore, an additional 20% of participants who initially had a cognitive screening score below phase 1 cut‐offs, but were not diagnosed with any form of cognitive impairment following phase 2 medical assessment and consensus panel review (i.e., false positive screening cases; n = 10), were randomly selected and included in the analysis. Only 20% of these participants were included as these participants scored lower on the screening tests, and we did not want to unduly bias the control group by oversampling from the group who screened positive at phase 1. The total normative sample of controls, therefore, consisted of participants that screened negative at phase 1 (n = 21), and those who were deemed to be cognitively intact at the consensus panel review (n = 10). That is, the normative sample comprises a 20% random sample of the total population, excluding those diagnosed with cognitive impairment or dementia.
Measures
Measures were selected by an expert panel consisting of clinical neuropsychologists (H. B. and K. R.), a neurologist (G. A. B.), and geriatrician (S. C.), and supported by the wider Aboriginal and non‐Aboriginal research panel. Given the dearth of validated cognitive tests for Aboriginal people, clinical assessments were selected to cover a range of cognitive domains relevant to dementia diagnosis in older people. These assessments were also piloted with n = 19 older Aboriginal people with and without cognitive impairment, including members of our local guidance group (community Elders) for their feedback on test appropriateness.
Addenbrooke's Cognitive Examination Revised
The Addenbrooke's Cognitive Examination Revised (ACE‐R) is a cognitive screening tool commonly used to assess MCI and dementia (Mioshi, Dawson, Mitchell, Arnold, & Hodges, Citation2006). It has been shown to be a sensitive and diagnostically accurate tool (Larner & Mitchell, Citation2014), with a cut‐off of 82 showing high sensitivity and specificity for dementia diagnosis (Mioshi et al., Citation2006). The ACE‐R has also been translated for use across different languages and cultures (see Habib & Scott, Citation2017). Participants are assessed on tasks relating to attention/orientation (18 points), memory (26 points), fluency (14 points), language (26 points), and visuospatial abilities (16 points), which form 5 sub‐scales; the overall test is scored out of 100.
Digit span
Participants were administered the Digit Span task from the WAIS‐III (Wechsler, Citation1997a). Digit Span Forward involved participants repeating strings of digits in the order that they were originally spoken. Digit Span Backward required participants to repeat the digits in reverse order. For both versions, the task was made more difficult by progressively increasing the number of digits in the sequence. The task was discontinued after incorrect responses for two trials of the same length. For both Forward and Backward conditions, outcomes included the longest string of digits correctly recalled (range of possible scores: 2–9 and 2–8 for Forward and Backward, respectively), and the total number of correct responses (range of possible scores: 0–16 and 0–14 for Forward and Backward, respectively).
Logical memory
A Logical Memory task was included, based on a modified version of Story A from the Wechsler Memory Scale‐III (WMS‐III) (Wechsler, Citation1997b), where some items were substituted to suit an Australian audience (Ivison, Citation1977). Early in the assessment section, participants were read a short story and asked to try and remember it. After hearing the story, participants were asked to repeat as much of the story as they could remember (Immediate Recall). Approximately 30-min later, they were asked to recall the story again, as closely as possible to the original recount (Delayed Recall). Participant responses at both time points were recorded verbatim by the administrator. Both of the recall tasks were scored out of 25. Following Delayed Recall, participants were asked a series of yes/no questions (15 total) about the story, to assess recognition memory (Recognition).
An overall Percent Recall score was computed by combining the raw scores for the Immediate and Delayed Recall, according to the formula: (Delayed Recall/Immediate Recall)*100. If the Immediate Recall score was 0, those participants scored 0 for the Percent Recall score. A maximum score of 100 was given if more details of the story were recalled on Delayed compared to Immediate Recall, in general accordance with WMS‐III guidelines. A total score for the Recognition task was also calculated.
Oral Trail making tests
Oral versions of the Trail Making Tests (A and B) were administered (Ricker & Axelrod, Citation1994). For Oral Trail Making A, participants were asked to count aloud from 1 to 25 as quickly as possible. For Oral Trail Making B, participants were asked to switch between numbers and letters, in the ascending order (i.e., 1‐A‐2‐B‐3‐C). For both versions, participants were timed using a stopwatch until they reached the number 25 (Oral Trail Making A), or the number 13 (Oral Trail Making B). If participants made an error, they were directed to go back to the last correct response and recommence the sequence. Total time in seconds was recorded for both versions. Oral Trail Making B was discontinued at 99-s, in line with the protocol for the Sydney Older Persons Study (Broe et al., Citation2007), to minimise the risk of participant distress.
Statistical analysis
Data were analysed using IBM SPSS v24 (IBM Corp., Armonk, NY). A subset of participants who originally screened negative were subsequently diagnosed with MCI at the consensus interview (n = 4 were included in the MCI group; n = 1 was diagnosed with “other cognitive impairment”). Participants with demographic data but no neuropsychological test data (n = 6, all diagnosed with severe dementia), and all individuals diagnosed with “other cognitive impairment” (n = 5), were excluded. Data from control participants who originally screened positive but were not randomised to the current sample (n = 38) were also excluded to avoid bias associated with including a larger percentage of lower‐scoring individuals.
Adjusted ACE‐R total and sub‐scores were calculated for participants with missing or incomplete items (n = 4, all diagnosed with dementia). Reasons for incomplete data included: dysphagia (n = 2); illiteracy (n = 1); and illiteracy and vision problems (n = 1). Adjusted ACE‐R sub‐scores were calculated according to the following formula: (score/maximum score possible for completed items)*total for that sub‐scale; the sum of these adjusted sub‐scores formed the total ACE‐R score. This method prevented exclusion of these participants from analyses, and retained the value of the original scores.
Demographic comparisons were carried out between control participants included in the final sample for these analyses (n = 31) and the remaining KGOWS participants who were not diagnosed with cognitive impairment (n = 217), to ensure the 20% sub‐sample was representative. Both groups were compared on urban/regional breakdown and sex (chi square test), age and education (Mann–Whitney U tests). Non‐parametric tests were used as age was positively skewed and both age and education had outliers in the larger sample not selected for this normative study.
Comparisons were also performed to determine whether the diagnostic groups differed on age or years of education. Three independent samples t‐tests were run to investigate differences between each of the diagnostic groups in terms of age. Due to the presence of outliers in the education variable (for the MCI and dementia groups), three Mann–Whitney U comparisons were run to determine whether there were statistical differences in education between the diagnostic groups. False discovery rate (FDR) correction (Benjamini & Hochberg, Citation1995) was applied across these tests to control for multiple comparisons.
Correlation analyses were also performed to determine whether age or education was associated with cognitive test scores within each group. Pearson correlations were performed for correlations between age and either ACE‐R Memory, Digit Span Forward and Logical Memory Percent Recall. Due to skew or outliers for the other cognitive variables, Spearman correlations were performed for the remaining correlations with age and education. FDR correction was applied across all correlations within each group.
Descriptive statistics included means, SDs, medians, interquartile ranges (IQR), and range of scores for all test variables. Percentile scores were calculated for the control group on each test variable, overall and stratified by education (low education = 0–9 years; high education = 10+ years).
Parametric statistics (three independent samples t‐tests) were used to compare the three groups (control vs dementia, control vs MCI, and dementia vs MCI) on the ACE‐R Memory, Digit Span Forward and Logical Memory Percent Recall variables. Non‐parametric statistics (Mann–Whitney U) were used to compare the three groups on variables that were skewed or had outliers (all variables except ACE‐R Memory, Digit Span Forward and Logical Memory Percent Recall). Again, FDR correction was applied across tests to account for multiple comparisons.
A Receiver Operating Characteristic (ROC) curve analysis was run to determine sensitivity, specificity, and the optimal ACE‐R score cut‐off for dementia. To determine this, ACE‐R scores from the control group and dementia group (excluding those with MCI) were included in the analysis. Youden's J statistic was calculated based on the formula: Sensitivity + Specificity – 1. The ACE‐R score with the largest Youden's J was identified as the optimal cut‐off for dementia.
RESULTS
Participants
A total of 104 participants were included in the analyses, classified as controls (n = 31), MCI diagnosis (n = 38), or dementia diagnosis (n = 35). The majority of participants with dementia were diagnosed with Alzheimer's disease (n = 16; 45.7%), followed by mixed dementia (n = 9; 25.7%), vascular dementia (n = 7; 20.0%) and dementia due to head trauma (n = 3; 8.6%). Of the total sample, 41 lived in urban areas and 63 in regional areas. All participants identified as Aboriginal with the exception of three participants who identified as both Aboriginal and Torres Strait Islander peoples. All participants spoke English, and only two participants identified that English was not their first language. Control group participants were representative and did not differ from other cognitively intact KGOWS participants (n = 217) on urban/regional breakdown (χ2 [1] = 0.002, p = 0.961), sex (χ2 [1] = 1.398, p = 0.237), age (Mann–Whitney U = 3,244.00, p = 0.748), or education (Mann–Whitney U = 3,169.50, p = 0.600). Demographic characteristics and the cognitive screening scores for the current study are shown in Table . Means, SDs, ranges, medians, and IQRs for each test variable are presented by cognitive status, in Table .
Table 1. Demographic and screening data according to each diagnostic group
Table 2. Descriptive statistics for major study variables according to the diagnostic group
Within the final sample, significant age differences were found between the control and dementia groups, t(58.02) = −3.38, p = 0.001, which remained significant after FDR correction. The age difference between the control and MCI groups, t(64.74) = −2.21, p = 0.031, was not significant after FDR correction (adjusted p‐value = 0.093). There was no age difference between the MCI and dementia groups, t(71) = 1.19, p = 0.237. There were no differences in education for any of the groups: control and MCI (Mann–Whitney U = 475.00, p = 0.165), control and dementia (Mann–Whitney U = 417.50, p = 0.105), or MCI and dementia (Mann–Whitney U = 649.00, p = 0.859).
Correlations between either age or education, and the cognitive test variables for each diagnostic group separately, revealed no significant associations (all p > 0.05) after FDR correction for multiple comparisons (see Supporting Information Table S1 for results). Given there were no associations between age or education and test performance, and an age difference between the control and dementia groups was the only difference found, these variables were not included as covariates in subsequent between‐group analyses.
Percentile scores for each test variable are reported for the control group in Table . Given the skewed distributions for some variables, percentile scores are likely more useful for clinical purposes. Scores have also been stratified by education. Overall, comparing individual scores with tests norms matched for education level is preferable. For instance, there is a discrepancy of six points on the ACE‐R total score at the 50th percentile (low education = 87 and high education = 93). If normative scores for the total sample were used, a test score of 87 would be considered closer to the 25th percentile; whilst a score of 93 would be considered in the 75th percentile. Whilst this example is more extreme than that seen for most other tests, and occasionally the percentile scores for the low education group are better than the high education group (although no correlations with education were seen for any test), this nevertheless highlights the importance of considering scores within the context of educational level, and not just for the normative sample as a whole.
Table 3. Control group percentile scores for main test variables, stratified by education
Sensitivity to cognitive impairment
To determine whether cognitive performance on each test differed between diagnostic groups, each of the groups were compared on test variables. Results are presented in Table . Differences between groups were generally found across the tests, where the control group performed better than both MCI and dementia groups, and the MCI group performed better than the dementia group. All statistically significant results (p < 0.05) remained significant after FDR correction. There were just three instances where the comparisons were non‐significant: the control and MCI groups did not differ on Digit Span Forward total correct or Oral Trail Making A total time (i.e., simple attention span/sequencing tasks); and the dementia and MCI groups did not differ on Logical Memory Recognition scores (both performed similarly poorly relative to the control group).
Table 4. Results from parametric (t‐tests) and non‐parametric (Mann–Whitney U) analyses comparing diagnostic groups on performance for test variables of interest
ROC curve analysis showed that the ACE‐R was able to discriminate the control and dementia groups (area under the curve = 0.986). An ACE‐R score of ≤82.5 was identified as the optimal cut‐off for dementia (sensitivity = 0.941, specificity = 0.935), based on Youden's J statistic = 0.877.
DISCUSSION
This study provides some of the first normative data for older Aboriginal Australians on a selection of common neuropsychological tests. Anecdotally, these tests were found to be generally acceptable with participants; and were sensitive to performance decrements for individuals with dementia or MCI, supporting their validity in this population. The normative data presented also allow for interpretation of test scores in the context of educational level, which may increase the fairness and/or sensitivity of clinical interpretations based on these data. Publishing these norms is not only integral to the interpretation of cognitive test scores in a research setting, but it also has important clinical applications (Dingwall et al., Citation2014; O'Connor, Citation1990). Given these tests are readily available and easy to administer, having norms specific to this population will assist with objective assessment of cognitive decline and monitoring of cognitive function over time.
In line with expectations, the control group performed better than both the MCI and dementia groups on the ACE‐R (total and sub‐scales), and the MCI group demonstrated better performance than those diagnosed with dementia. The optimal cut‐off for dementia diagnosis was identified as an ACE‐R score of ≤82.5. These findings are in accordance with research from the UK reporting better performance across the ACE‐R total and sub‐scores (excluding Attention and Visuospatial) in controls; and better performance in MCI compared to dementia; with a cut‐off of 82 indicative of dementia (Mioshi et al., Citation2006). Substantial differences in all sub‐scores in the current study, including Attention and Visuospatial, could be due to slightly poorer performance (linked to lower education) than participants in Mioshi et al. (Citation2006). However, when stratified by education, ACE‐R performance for those with higher levels of education in the current normative sample of Aboriginal Australians is consistent with that of the control group from Cambridge, UK (Mioshi et al., Citation2006). The current ACE‐R results also generally align with those reported in a non‐Aboriginal sample from Sydney, Australia (Terpening, Cordato, Hepner, Lucas, & Lindley, Citation2011). Control group results reported in the current study for ACE‐R total and sub‐scores were equivalent to or slightly higher (within 1 point) than those reported by Terpening et al. (Citation2011). However, unlike these previous memory clinic studies (Mioshi et al., Citation2006; Terpening et al., Citation2011), the current normative data are derived from a representative sample of a population‐based cohort of older people, further strengthening the reliability of our results.
Digit span performance was better in the control than the MCI or dementia groups, and better in MCI than dementia, with one exception: there was no significant difference between the control and MCI groups in terms of Digit Span Forward. Task difficulty can explain this result, as Digit Span Forward relies less on higher order executive functions than does Digit Span Backward. This is supported by Griffith et al. (Citation2006), who reported no Digit Span differences between control and MCI groups; although no distinction was made between the Forward and Backward subtests. Other research has shown that Digit Span performance (Forward and Backward) differs between control and MCI groups (Kessels, Molleman, & Oosterman, Citation2011; Kessels, Overbeek, & Bouman, Citation2015), control and dementia groups (including Alzheimer's disease and Lewy body dementia) (Calderon et al., Citation2001; Kessels et al., Citation2015), and MCI and dementia groups (Kessels et al., Citation2015). On the other hand, null results have been found between all groups (Kramer et al., Citation2006), and the MCI and dementia groups specifically (Kessels et al., Citation2011). Despite these inconsistencies, the current findings extend previous work by demonstrating performance differences in an Aboriginal Australian sample.
Consistent with the Digit Span results, control participants performed best on Oral Trail Making, with the poorest performance in those with dementia; however, there was no significant difference in Oral Trail Making A between the control and MCI groups. The Oral Trail Making Test results for the control group are slightly poorer than data for 59–79-year olds presented by Mrazik, Millis, and Drane (Citation2010), particularly for Oral Trail Making B; but differences in average education levels could contribute to this discrepancy. Differences in Oral Trail Making A and B for control and dementia groups have been found in a previous Australian study (Kowalczyk, McDonald, Cranney, & McMahon, Citation2001). These findings also replicate a study demonstrating control, MCI, and dementia group performance differences with the exception of the control and MCI comparison, on Oral Trail Making A (Bastug, Ozel‐Kizil, Altintas, Kirici, & Altunoz, Citation2013). It should be noted that Trail Making A, particularly in the oral format, is less of a test of executive functioning, and is rather a control task for Trails B that assesses the speed of reciting an overlearned sequence, which appears not to be impaired in MCI.
For Logical Memory, best performance was observed in the control group and poorest performance in the dementia group; although there was no difference between the MCI and dementia groups in terms of the Recognition task. Given memory is typically impaired in both MCI and dementia (Dubois & Albert, Citation2004; Perneczky et al., Citation2006), this could explain the lack of difference in recognition memory. There is support for better performance in controls than those with MCI (Griffith et al., Citation2006; Grundman et al., Citation2004; Rabin et al., Citation2009; Ribeiro, de Mendonca, & Guerreiro, Citation2006) or dementia (Calderon et al., Citation2001; Kowalczyk et al., Citation2001) on Logical Memory (particularly the delayed recall task). Baseline performance differences have even been found longitudinally between individuals who later converted to Alzheimer's disease versus those who did not (Elias et al., Citation2000; Rabin et al., Citation2009). These findings support the utility of the Logical Memory task in assessing cognitive decline in older Aboriginal Australians. Using stories might enhance the acceptability of memory assessments in this population, and there could be opportunities to develop culturally specific adaptations of the Logical Memory test, for more widespread use across Aboriginal Australians. However, given the long administration time (including 30-min delayed recall), this type of test is less readily administered in primary care settings and is more appropriate as part of neuropsychological assessment in specialist clinics or research settings.
Strengths and limitations
This article provides the first normative data for older Aboriginal Australians on a range of commonly used cognitive assessments. This is important given that the Aboriginal Australian population is ageing (ABS, Citation2014), and dementia and cognitive decline are more prevalent in this population (Radford et al., Citation2015a; Smith et al., Citation2008). Hence, there is an increased need for reliable, validated assessments to monitor cognitive decline. A strength of this study is the generalisability to many Aboriginal Australians, the majority of whom live in urban and regional areas (ABS, Citation2016). Compared with the broader community‐based KGOWS cohort, the current sub‐sample was consistent in terms of urban/regional breakdown, sex, age, and education. Although dementia is unrecognised as a medical condition for some Aboriginal people (Arkles et al., Citation2010), and despite the assessments used in the current study being considered more “mainstream,” these assessment tools are still relevant for Aboriginal people (particularly in an urban/regional context) who also have strong ties to their Aboriginal culture. Another strength is the reporting of norms for the Oral Trail Making Tests, for which robust normative data is not yet available for any population (Kaemmerer & Riordan, Citation2016). Given that older people may have visual and motor impairments that can affect performance on the written version of Oral Trail Making, it is important to consider the utility of alternative measures that are less impacted by physical capabilities.
Conversely, a limitation of the study is that the Oral Trail Making test may not be equivalent to the conventional written version of the task, although they likely both reflect set‐shifting abilities (Mrazik et al., Citation2010). However, this method of administration was chosen over the written version due to the importance of administering tests in ways that are more likely to be culturally safe (Dingwall et al., Citation2014). Another limitation is the relatively small sample size, as normative data are ideally derived from large samples. These results may also not be generalisable to remote Aboriginal communities or Aboriginal people who do not speak English fluently. However, this sample was randomly selected from, and highly representative of, a larger cohort of older Aboriginal Australians from urban and regional communities, successfully recruited using culturally appropriate population‐based methods (Radford et al., Citation2015a); and reflects urban and regional Aboriginal communities more broadly. Moreover, the current normative sample only included individuals who were confirmed to be cognitively intact following comprehensive assessment and clinical consensus review. It should also be considered that clinical diagnosis was not completely independent of cognitive testing, and the neuropsychological tests examined in this study formed part of the holistic clinical consensus review. However, given the lack of normative data for Aboriginal Australians, cognitive test performance only partially informed the diagnosis, in a largely qualitative way; this is evident in the variation of performance within the groups, as well as the overlap in performance across the groups for all of the tests.
No associations were found between age or education and any of the cognitive tests. This could be due to the relatively constrained age range within the control group (aged 60–74-years). Inclusion of cognitively intact older people aged over 75 may have elucidated age‐effects; however, in this community‐based cohort, the majority were aged 70-years or younger (71%), and the oldest participants were more likely to be diagnosed with dementia or MCI compared to younger participants. In terms of education, there was a small number of participants with higher levels of education in this sample, and only five cases in the control group of education >12-years; this may have reduced associations with cognitive performance.
Further research to enhance the cultural relevance, reliability, and availability of validated cognitive assessment tools for Aboriginal Australians is crucial (as it is in Indigenous populations globally). This may include development of novel culturally specific neuropsychological tests. However, high‐quality normative studies with certain existing tools (as appropriate) are also important for increased culturally fair interpretation of test scores and cross‐cultural inclusion in major research studies on the prevention and treatment of cognitive decline and dementia.
Conclusion
This study provides the first normative data for older Aboriginal Australians on a range of conventional cognitive tests, and has demonstrated the consistency of performance differences between cognitively intact older people and those with MCI and dementia when compared to previous literature in non‐Aboriginal samples. Having appropriate norms for comparison with individual scores is imperative for interpreting cognitive test performance, and this article provides data from a representative sample of older Aboriginal people. These normative data can be used in future clinical and research work, and may assist with culturally relevant assessment of older Aboriginal Australians.
raup_a_12098938_sm0001.docx
Download MS Word (15.2 KB)ACKNOWLEDGEMENTS
This study was supported by an Australian National Health and Medical Research Council (NHMRC) project grant (510347). L.L. is supported by a Serpentine Foundation Fellowship. K.R. is supported by a NHMRC‐ARC Dementia Research Development Fellowship (1103312). K.D. is supported by a NHMRC Career Development Fellowship (1105106).
This work relied on the support of many individuals and organisations. We are extremely grateful to our participants, local Elder guidance groups, KGOWS Aboriginal Reference Group, the Aboriginal Health and Medical Research Council, and our Aboriginal community partners (Durri Aboriginal Corporation Medical Service and Booroongen Djugun in Kempsey, Darrimba Maarra Aboriginal Health Clinic in Nambucca, Galambila Aboriginal Health Service in Coffs Harbour, La Perouse Local Aboriginal Land Council, La Perouse Aboriginal Community Health Centre, and Tharawal Aboriginal Corporation in Campbelltown). We recognise the work of our research team including Aboriginal research assistants, investigators, project officers and interviewers, medical doctors, and knowledge translation and support team (Koori Dementia Care Project).
Additional information
Funding
Notes
Funding information National Health and Medical Research Council, Grant/Award Number: 1105106510347; NHMRC‐ARC Dementia Research Development Fellowship, Grant/Award Number: 1103312; Serpentine Foundation Fellowship
REFERENCES
- ABS. (2014). 3238.0—Estimates and projections, aboriginal and Torres Strait Islander Australians, 2001 to 2026. Retrieved from http://www.abs.gov.au/ausstats/[email protected]/Products/C19A0C6E4794A3FACA257CC900143A3D?opendocument
- ABS. (2016). 3238.0.55.001—Estimates of aboriginal and Torres Strait Islander Australians, June 2011. Retrieved from http://www.abs.gov.au/ausstats/[email protected]/mf/3238.0.55.001
- Anstey, K. J., Kiely, K. M., Booth, H., Birrell, C. L., Butterworth, P., Byles, J., … Gibson, R. (2011). Indigenous Australians are under‐represented in longitudinal ageing studies. Australian & New Zealand Journal of Public Health, 35, 331–336. https://doi.org/10.1111/j.1753-6405.2011.00727.x
- Arkles, R. S., Jackson pulver, L. R., Robertson, H., Draper, B., Chalkley, S., & Broe, G. A. (2010). Ageing, cognition and dementia in Australian Aboriginal and Torres Strait Islander Peoples. Neuroscience Research Australia and Muru Marri Indigenous Health Unit, University of New South Wales.
- Australian Institute of Health and Welfare. (2012). Dementia in Australia. Canberra, Australian Capital Territory, Australia: AIHW.
- Bastug, G., Ozel‐kizil, E. T., Altintas, O., Kirici, S., & Altunoz, U. (2013). Oral Trail making task as a discriminative tool for different levels of cognitive impairment and normal aging. Archives of Clinical Neuropsychology, 28, 411–417. https://doi.org/10.1093/arclin/act035
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological), 57(1), 289–300.
- Broe, G. A., Grayson, D. A., Creasey, H., Halliday, G., Harding, A., Brooks, W. S., … Pynoos, J. (2007). Sydney older persons study, 2000–2003. [Computer file]. Canberra, Australian Capital Territory, Australia: Australia Social Science Data Archive, The Australian National University.
- Calderon, J., Perry, R. J., Erzinclioglu, S. W., Berrios, G. E., Dening, T. R., & Hodges, J. R. (2001). Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 70, 157–164.
- Davidson, G. (1995). Cognitive assessment of indigenous Australians: Towards a multiaxial model. Australian Psychologist, 30(1), 30–34. https://doi.org/10.1080/00050069508259601
- Dingwall, K. M., & Cairney, S. (2010). Psychological and cognitive assessment of Indigenous Australians. Australian & New Zealand Journal of Psychiatry, 44(1), 20–30.
- Dingwall, K. M., Lindeman, M. A., & Cairney, S. (2014). You've got to make it relevant: Barriers and ways forward for assessing cognition in Aboriginal clients. BMC Psychology, 2(13), 1–11. https://doi.org/10.1186/2050-7283-2-13
- Dubois, B., & Albert, M. (2004). Amnestic MCI or prodromal Alzheimer's disease. Lancet Neurology, 3, 246–248.
- Elias, M. F., Beiser, A., Wolf, P. A., Au, R., White, R. F., & D'agostino, R. B. (2000). The preclinical phase of Alzheimer disease: A 22‐year prospective study of the Framingham cohort. Archives of Neurology, 57, 808–813.
- Folstein, M. F., Folstein, S. E., & Mchugh, P. R. (1975). Mini‐mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198.
- Griffith, H. R., Netson, K. L., Harrell, L. E., Zamrini, E. Y., Brockington, J. C., & Marson, D. C. (2006). Amnestic mild cognitive impairment: Diagnostic outcomes and clinical prediction over a two‐year time period. Journal of the International Neuropsychological Society, 12, 166–175. https://doi.org/10.1017/0S1355617706060267
- Grundman, M., Petersen, R. C., Ferris, S. H., Thomas, R. G., Aisen, P. S., Bennett, D. A., … Thal, L. J. (2004). Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology, 61, 59–66.
- Habib, N., & Scott, J. (2017). Systematic review of the diagnostic accuracy of the non‐English versions of Addenbrooke's cognitive examination—Revised and III. Aging & Mental Health, 1–8. https://doi.org/10.1080/13607863.2017.1411882
- Ivison, D. J. (1977). The Wechsler memory scale: Preliminary findings toward an Australian standardisation. Australian Psychologist, 12, 303–312. https://doi.org/10.1080/00050067708254291
- Kaemmerer, T., & Riordan, P. (2016). Oral adaptation of the trail making test: A practical review. Applied Neuropsychology: Adult, 23, 384–389. https://doi.org/10.1080/23279095.2016.1178645
- Kessels, R. P. C., Molleman, P. W., & Oosterman, J. M. (2011). Assessment of working‐memory deficits in patients with mild cognitive impairment and Alzheimer's dementia using Wechsler's working memory index. Aging Clinical and Experimental Research, 23, 487–490.
- Kessels, R. P. C., Overbeek, A., & Bouman, Z. (2015). Assessment of verbal and visuospatial working memory in mild cognitive impairment and Alzheimer's disease. Dementia Neuropsychologia, 9, 301–305. https://doi.org/10.1590/1980-57642015DN93000014
- Kowalczyk, A., Mcdonald, S., Cranney, J., & Mcmahon, M. (2001). Cognitive flexibility in the normal elderly and in persons with dementia as measured by the written and oral trail making tests. Brain Impairment, 2(1), 11–21.
- Kramer, J. H., Nelson, A., Johnson, J. K., Yaffe, K., Glenn, S., Rosen, H. J., & Miller, B. L. (2006). Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 22, 306–311. https://doi.org/10.1159/000095303
- Larner, A. J., & Mitchell, A. J. (2014). A meta‐analysis of the accuracy of the Addenbrooke's cognitive examination (ACE) and the Addenbrooke's cognitive examination‐revised (ACE‐R) in the detection of dementia. International Psychogeriatrics, 26, 555–563. https://doi.org/10.1017/S1041610213002329
- Li, S. Q., Guthridge, S. L., Aratchige, P. E., Lowe, M. P., Wang, Z., Zhao, Y., & Krause, V. (2014). Dementia prevalence and incidence among the Indigenous and non‐Indigenous populations of the Northern Territory. The Medical Journal of Australia, 200, 465–469. https://doi.org/10.5694/mja13.11052
- Logiudice, D., Smith, K., Thomas, J., Lautenschlager, N. T., Almeida, O. P., Atkinson, D., & Flicker, L. (2006). Kimberley indigenous cognitive assessment tool (KICA): Development of a cognitive assessment tool for older Indigenous Australians. International Psychogeriatrics, 18, 269–280. https://doi.org/10.1017/S1041610205002681
- Logiudice, D., Strivens, E., Smith, K., Stevenson, M., Atkinson, D., Dwyer, A., … Flicker, L. (2010). The KICA screen: The psychometric properties of a shortened version of the KICA (Kimberley Indigenous Cognitive Assessment). Australasian Journal of Ageing, 30, 215–219. https://doi.org/10.1111/j.1741-6612.2010.00486.x
- Mckhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas, C. H., … Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7, 263–269. https://doi.org/10.1016/j.jalz.2011.03.005
- Mioshi, E., Dawson, K., Mitchell, J., Arnold, R., & Hodges, J. R. (2006). The Addenbrooke's cognitive examination revised (ACE‐R): A brief cognitive test battery of dementia screening. International Journal of Geriatric Psychiatry, 21, 1078–1085. https://doi.org/10.1002/gps.1610
- Mrazik, M., Millis, S., & Drane, D. L. (2010). The Oral Trail making test: Effects of age and concurrent validity. Archives of Clinical Neuropsychology, 25, 236–243. https://doi.org/10.1093/arclin/acq006
- O'connor, P. J. (1990). Normative data: Their definition, interpretation, and importance for primary care physicians. Family Medicine, 22, 307–311.
- Perneczky, R., Pohl, C., Sorg, C., Hartmann, J., Tosic, N., Grimmer, T., … Kurz, A. (2006). Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. International Journal of Geriatric Psychiatry, 21, 158–162. https://doi.org/10.1002/gps.1444
- Rabin, L. A., Pare, N., Saykin, A. J., Brown, M. J., Wishart, H. A., Flashman, L. A., & Santulli, R. B. (2009). Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimers disease. Aging, Neuropsychology, and Cognition, 16, 357–376. https://doi.org/10.1080/13825580902825220
- Radford, K., & Mack, H. A. (2012). Dementia screening for urban aboriginal Australians: The modified Kimberly Indigenous Cognitive Assessment (mKICA). Sydney, New South Wales, Australia: Dementia Collaborative Research Centres.
- Radford, K., Mack, H. A., Draper, B., Chalkley, S., Daylight, G., Cumming, R., … Broe, G. A. (2015a). Prevalence of dementia in urban and regional Aboriginal Australians. Alzheimer's & Dementia, 11, 271–279. https://doi.org/10.1016/j.jalz.2014.03.007
- Radford, K., Mack, H. A., Draper, B., Chalkley, S., Delbaere, K., Daylight, G., … Broe, G. A. (2015b). Comparison of three cognitive screening tools in older urban and regional Aboriginal Australians. Dementia and Geriatric Cognitive Disorders, 40, 22–32. https://doi.org/10.1159/000377673
- Radford, K., Mack, H. A., Robertson, H., Draper, B., Chalkley, S., Daylight, G., … Broe, G. A. (2014). The Koori Growing Old Well Study: Investigating aging and dementia in urban Aboriginal Australians. International Psychogeriatrics, 26, 1033–1043. https://doi.org/10.1017/S1041610213002561
- Ribeiro, F., de Mendonca, A., & Guerreiro, M. (2006). Mild cognitive impairment: Deficits in cognitive domains other than memory. Dementia and Geriatric Cognitive Disorders, 21, 284–290. https://doi.org/10.1159/000091435
- Ricker, J. H., & Axelrod, B. N. (1994). Analysis of an oral paradigm for the Trail Making Test. Assessment, 1(1), 47–51.
- Smith, K., Flicker, L., Dwyer, A., Marsh, G., Mahajani, S., Almeida, O. P., … Logiudice, D. (2009). Assessing cognitive impairment in Indigenous Australians: Re‐evaluation of the Kimberley Indigenous Cognitive Assessment in Western Australia and the Northern Territory. Australian Psychologist, 44(1), 54–61. https://doi.org/10.1080/00050060802563463
- Smith, K., Flicker, L., Lautenschlager, N. T., Almeida, O. P., Atkinson, D., Dwyer, A., & Logiudice, D. (2008). High prevalence of dementia and cognitive impairment in Indigenous Australians. Neurology, 71, 1470–1473.
- Storey, J. E., Rowland, J. T., Basic, D., Conforti, D. A., & Dickson, H. G. (2004). The Rowland Universal Dementia Assessment Scale (RUDAS): A multi‐cultural cognitive assessment scale. International Psychogeriatrics, 16, 13–31.
- Terpening, Z., Cordato, N. J., Hepner, I. J., Lucas, S. K., & Lindley, R. I. (2011). Utility of the Addenbrooke's Cognitive Examination—Revised for the diagnosis of dementia syndromes. Australasian Journal of Ageing, 30, 113–118. https://doi.org/10.1111/j.1741-6612.2010.00446.x
- Wechsler, D. (1997a). WAIS‐III, Wechsler Adult Intelligence Scale: Administration and scoring manual. San Antonio, TX: The Psychological Corporation.
- Wechsler, D. (1997b). Wechsler Memory Scale—Third edition manual. San Antonio, TX: The Psychological Corporation.
- Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., … Petersen, R. C. (2004). Mild cognitive impairment—beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. Journal of International Medicine, 256, 240–246.
- Zann, S. (1994). Identification of support, education and training needs of rural/remote health care service providers involved in dementia care. Townsville, Queensland, Australia: Northern Regional Health Authority.