Abstract
Objective: Multiple myeloma (MM) is a plasma cell malignancy comprising 15% of hematological malignancies. Many studies have assessed the relationship between free radicals and tumor progression or cancer risk. We aimed to evaluate the antioxidant activity of paraoxonase 1 (PON1), arylesterase (ARE), and 8-isoprostane in patients with stage I MM.
Materials and methods: Spectrophotometric assays of serum PON1 and ARE activities in addition to serum 8-isoprostane level were performed in 34 patients newly diagnosed with stage I MM as compared to 35 age- and sex-matched individuals who comprised the healthy control group.
Results: A significant reduction was found in the activities of PON1 and ARE (for both, P < 0.001) in the patient group. The ratio of PON1/high-density lipoprotein was significantly lower in the MM patient group than in the control group (P < 0.001), while 8-isoprostane levels compared with the control group were significantly higher (P < 0.001), observations that may indicate an increase in oxidative stress in stage I MM patients.
Conclusion: A decrease in PON1 activity and increase in 8-isoprostane serum activities in patients may indicate the importance of lipid peroxidation in MM disease. Oxidative stress and especially lipid peroxidation could reduce the antioxidant activity of PON1 and ARE in MM patients and could be considered as factors in the pathogenesis of MM disease.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by the accumulation of plasma cells in the bone marrow. MM patients suffer from symptoms such as severe bone pain, anemia, renal failure, and recurrent infections due to incomplete immunoglobulin secretion.Citation1 Formerly, the mean age of patients with MM was 65 years. However, during the past 60 years the mean age of patients with MM has decreased to less than 55 years.Citation2 Although the exact etiology is unknown among various mechanisms that may have roles in the etiology of MM, factors such as genetic damage, environmental factors, ultraviolet rays, inflammation, and long-term activity, and stimulation of the reticuloendothelial system, have been found to contribute to the MM disease.Citation3
Oxidative stress, as a primary factor, has also been considered in the initial stages of carcinogenesis. Reactive oxygen species can damage the cellular structure of proteins, lipids, membranes, and DNA. Increased oxidative stress could increase the risk of carcinogenesis. Moreover, reduced levels of the antioxidant activities that act as a barrier against these reactive oxygen species could also increase the risk of carcinogenesis.Citation4 Various studies have shown the effect and key role of reactive oxygen species in the initiation of inflammation and tumor progression. Malignant cancer cells may decrease the antioxidant defense system by creating reactive oxygen species. In turn, a decrease in the antioxidant defense system is associated with initiation of malignant disease phenotypes.Citation5
F2-isoprostanes, a group of bioactive prostaglandin F2-like compounds generated by the oxidatively catalyzed metabolism of arachidonic acid, are reliable markers of lipid peroxidation in vivo. The 8-isoprostane (8-isoprostaglandin F2alpha, the major F2-isoprostane) is a compound produced from lipid peroxidation and is an important index for oxidative stress assessments. The 8-isoprostane is produced during oxidative stress by a non-enzymatic method from membranous phospholipids, glycolipids, and arachidonic acid.Citation6 In animal models, elevated lipid peroxidation levels are closely related to carcinogenesis.Citation7 The human serum paraoxonase-1 (PON1) was initially identified during high-density lipoprotein (HDL) electrophoresis as a component of HDL. This 44 kDa lipophilic enzyme can reversibly bind to its organophosphate substrate and hydrolyze free radicals and also has the ability to hydrolyze aromatic esters such as phenylacetate. The term arylesterase (ARE) was introduced for the enzyme that hydrolyses both compounds. PON1 is synthesized by the liver, accumulates in HDL, and enhances the antioxidant activity of HDL. PON1 requires calcium ions to be able to properly function and prevent lipid and LDL peroxidation.Citation8 In patients suffering from pancreas, esophagus, lung, stomach tumors, and breast cancer, the activity of this enzyme showed a significant reduction.Citation9–Citation13 Studies that have evaluated PON1 and ARE status in patients with MM are scarce. In addition, there is no study of serum 8-isoprostane levels in patients with MM. We therefore measured 8-isoprostane as a biomarker for lipid peroxidation in stage I MM in comparison to a healthy group for better monitoring of antioxidant/oxidative stress balance in the early diagnosis of stage I MM.
Material and methods
Collection of blood samples
We designed a case–control study on stage I MM patients. The study was approved by the Ethical Committee of Urmia Medical University (Faculty of Medicine, Department of Clinical Biochemistry, Urmia, Iran). Informed consents were obtained from patients prior to the study. The study protocols and procedures were approved by Urmia Medical University. A total of 69 subjects, including 34 patients (mean age: 69 ± 5.0 years, 22 males and 12 females) were newly diagnosed with stage I MM and 35 healthy controls (mean age: 71 ± 7.0 years, 22 males and 13 females), were enrolled in this study. The patients were diagnosed as having stage I MM after serum protein electrophoresis and immunotyping by capillary zone electrophoresis (Capillarys®, Sebia, France) using a high-resolution buffer kit (Sebia, Cedex, France). In comparison with conventional serum protein electrophoresis, the latter is a reproducible, rapid, and reliable serum electrophoresis in clinical chemistry that makes possible it to fractionate and analyze the proteins. A long peak in the gamma (>30%) or beta region and observance of kappa and lambda chains with respect to IgA, IgM, and IgG immunoglobulins indicated malignancy. Staging was done using international staging systems based on serum beta2-microglobulin and albumin levels: patients with serum beta2-microglobulin less than 3.5 mg/l were considered as having MM (Chemiluminescence, Diasorin, Liaison, Italy; detection limit: 0.12 mg/l and CV = 3.9%).Citation14 IgG kappa in 22 patients, IgG lambda in 5 patients, IgA kappa in 2 patients, IgA lambda in 2 patients, and IgM kappa in 3 patients. Exclusion criteria were patients with a history of chemotherapy, antioxidant consumption, cardiovascular and renal disease, diabetes mellitus, and chronic infections. Venous blood samples were obtained after a 12 hours fast. The serum was separated after being maintained at room temperature for 15 minutes via centrifugation at 4000 rpm (Hermle Z360 K, Japan).
Assessment of PON1 and ARE activity
The paraoxonase activity of PON1 was measured using paraoxon (O, O-diethyl-o-p-nitro-phenylphosphate; Sigma Chemical Co, UK) as the substrate and measured by increases in the absorbance at 412 nm due to the formation of 4-nitrophenol. Briefly, the activity was measured at 25°C by adding 30 µl serum buffer Tris–HCl (100 mmol, pH 8.0) containing 2 mM paraoxon and 2 mM CaCl2. The rate of generation of 4-nitrophenol was determined at 412 nm with a spectrophotometer (Techcomp 8500 II UV/VIS, China). A unit of PON1 activity was defined as 1 μmol of p-nitrophenol formed per minute and was calculated by using the molar extinction coefficient 17 100 M−1cm−1 and the enzyme activity was expressed in U/l. The coefficient of variation (CV) for 15 measurements was 3.4%. The ARE activity was evaluated using phenyl acetate (Sigma Co, London, UK) as substrate and the activity was measured at 25 °C in buffer Tris–HCl (20 mM, pH: 8.0), containing 4 mM phenyl acetate and 1 mM CaCl2. The hydrolysis rate of the substrate was determined by the production of phenol with a photospectrometry method at a wavelength of 270 nm. Enzyme activity was calculated using the molar extinction coefficient of 1310 M−1cm−1. The results are reported as kU/l.
Determination of serum 8-isoprostane levels
8-Isoprostane levels were determined using an ELISA kit (Cayman Chemicals, Ann Arbor, MI, USA) as instructed by the manufacturer. Determination of 8-isoprostane levels in each sample was conducted according to the kit protocol, using the mouse anti-rabbit IgG-coated plate provided in the kit and a standard curve of 8-isoprostane. The amounts were reported as pg/ml, the intra-assay, and inter-assay CV were 7.2 and 15.5%, with a detection limit of approximately 2.7 pg/ml. The serum HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), calcium, creatinine, and total protein levels were measured directly with standard laboratory techniques using commercially available assay kits (Biosystem, Barcelona, Spain).
Statistical analysis
Data were analyzed using the SPSS software, version 18 (SPSS, Inc., Chicago, IL, USA). Data are shown as mean ± SD. Student's unpaired t-test was used to assess the significance of differences between groups (if homogeneity of variables was assumed) and Pearson's correlation coefficient was carried out as appropriate to analyze the relationship between PON1, ARE activity, and 8-isoprostane levels. A value of P < 0.05 was considered as demonstrating statistically significant differences.
Results
The clinical profile and laboratory findings of the MM patients and control group are summarized in Table . No significant difference was seen between the two groups with respect to age and sex. The serum PON1 and ARE activities differed significantly in the case groups compared to the control group (P < 0.0001) (Figs. and ). As shown, significant decreases in the PON1 and ARE activities were observed. The serum 8-isoprostane levels were elevated in the patients (P < 0.001) (Fig. ). Moreover, HDL-C levels showed a significant reduction in the patient group compared to the control group (P < 0.001) (Table ). When the PON1 activity was standardized with respect to HDL concentration, the PON1/HDL ratio was found to be significantly lower in MM patients as compared to the control group. Serum LDL-C level was significantly higher in patients (P < 0.001) (Table ). No significant correlations were identified between beta2-microglobulin, hemoglobin, creatinine, calcium levels and PON1, ARE, or 8-isoprostane levels.
Figure 1 Serum PON1 activity in MM patients as compared with controls (117.35 ± 14.6 vs. 205.91 ± 19.32 U/l); mean ± SD.
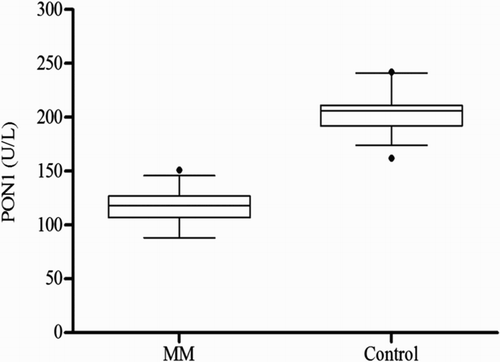
Figure 2 Serum ARE activity in MM patients as compared with controls (68.79 ± 10.95 vs. 161.34 ± 20.47 kU/l); mean ± SD.
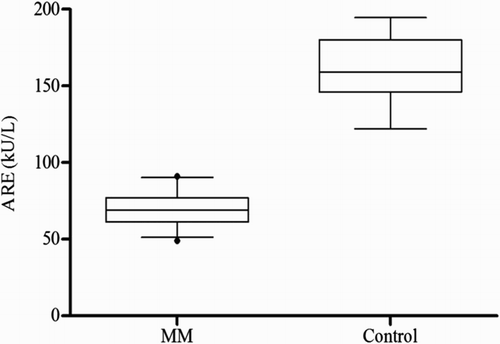
Figure 3 Serum 8-isoprostane in MM patients as compared with controls (68.73 ± 12.83 vs. 48.31 ± 9.9 pg/ml); mean ± SD.
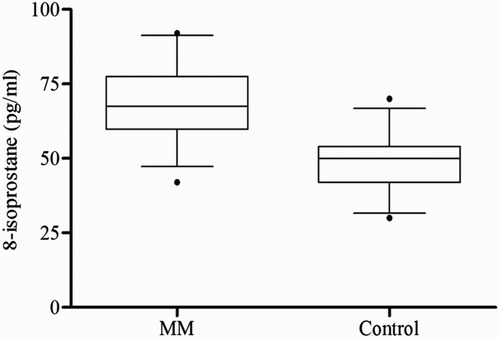
Table 1 Demographic features and biochemical parameters in control subjects and MM patients
Discussion
Increased amounts of reactive oxygen species may lead to changes in oxidant/antioxidant balance toward oxidative stress. Increased fluxes of free radicals are associated with biomolecule damage and play a key role in the initiation of various complications such as cardiovascular diseasesCitation15 and cancer.Citation16 The final products of lipid peroxidation have an important role in activating oncogenes by interfering with the balance between the antioxidant/oxidative stress system and through expression of some genes that contribute to cancer initiation and progression.Citation17 We found that the antioxidant activities of PON1 and ARE were significantly lower in MM patients compared to the control group. To evaluate whether the observed reduction of PON1 activity was due to the reduced HDL concentration (PON/HDL), we determined that the standardized enzyme activities were lower in the patient group compared to the control group. These data indicate that PON1 activity changes are not entirely dependent on HDL concentration in this study group. This reduction in PON1 activity could be related to enhanced lipid peroxidation, since lipid peroxidation is reported to inhibit PON1 activity. We observed a significant increase in serum 8-isoprostane, which is a lipid peroxidation biomarker, while serum PON1 and ARE activities were decreased in MM patients compared to the control group. This is the first report on 8-isoprostane levels in patients with stage I MM.
It has become clear that measurement of F2-isoprostanes provides a valuable and reliable approach for assessing oxidative stress in vivo. Measurement of F2-isoprostanes has firmly established the occurrence of oxidative stress in a wide variety of disease states.Citation18 In our study, there was an increase in serum 8-isoprostane level in MM patients compared with controls. These results concur with a previous study by Khadem-Ansari et al.,Citation19 who also found an increase in esophageal squamous cell carcinoma patients. The study results are concordant with the study of Dalaveris et al.,Citation20 who also found increased serum 8-isoprostane level in exhaled breath condensate and serum of patients with lung cancer.
Serum PON1 binds to HDL and contributes to the detoxification of compounds such as paraoxon and carcinogenic lipid soluble radicals from lipid peroxidation. There was a reduction in the serum HDL-C levels in the MM patients compared to the control group. Several studies have pointed out abnormalities in oxidative stress and antioxidant activity in MM patients.Citation21 Moreover, Sharma et al. investigated the enzymatic antioxidant activity and non-enzymatic antioxidant levels in patients with stage II of MM. They found a significant decreased in the redox potential in MM patients.Citation22 Lodh and co-workers also reported decreased antioxidant levels. They examined the levels of MD and SOD in 20 cases of MM patients in stage II and III.Citation23 Gangemi et al. also analyzed the serum levels of protein oxidation markers in order to quantify the oxidative stress in MM patients and in patients affected by monoclonal gammopathy of uncertain significance. They indicated that protein oxidation was significantly increased in MM patients compared with controls.Citation24 Ellidag and colleagues also investigated serum PON1 and ARE activities in MM patients and showed a decrease in enzyme activities of PON1 and ARE in these patients. These authors did not mention the stage of disease and lipid peroxidation status, which could affect PON1 activity as part of the lipid peroxidation scavenger system. Moreover, 8-isoprostane levels have not been studied in MM.Citation25
In other studies on the reduction of PON1 activity in tumors, researchers have investigated the activity of this enzyme in patients with esophageal squamous,Citation26 pancreatic,Citation27 and gastric cancerCitation9 and found low HDL and PON1 levels. Elkiran et al. found even lower levels of PON1 activity in patients with lung cancer.Citation10 However, they did not specify the stage of disease. Krzystek-Korpacka and co-workers found that reduced PON1 activity was a marker for the metastasis stage of esophageal cancer to lymph nodes.Citation11 One of the important capabilities of HDL is its function as the carrier and reservoir of PON1, which inhibits and restricts the accumulation of oxidized phospholipids. In evaluating lipid profile, HDL levels had a significant decrease in patients compared with the control group, which also has been reported in Akcay et al. study.Citation9 However, in a study on patients with lung cancer, no significant difference was found between HDL levels in the two studied groups.Citation28
There was no correlation between beta2-microglobulin, hemoglobin, creatinine, calcium, and 8-isoprostane levels and PON1 or ARE activities in the first stage of disease or stage I MM based on these findings, the change in the antioxidant/oxidative stress balance system did not correlate with symptoms of disease. The mechanism for the observed decrease in PON1 and ARE enzyme activity and the increased 8-isoprostane levels in patients with MM is still unclear, though it might be due to increased lipid peroxidation, since lipid oxidation reduces the enzyme activity of PON1. This reduction could be because of increased ROS levels in cancer, which is in turn considered in the pathogenesis of MM via lipid peroxidation. Malnutrition, inflammation, and cachexia are fairly common findings in cancer patients. Malnutrition and cachexia could affect protein synthesis and suppress PON1 production.Citation29 Serum PON1 activity has been shown to be lower during inflammation.Citation30 The decrease in PON1 activity thus may be in response to inflammation in patients with MM. Previous studies on antioxidant activity in patients with different stages of MM confirm the decreased activity of antioxidants and involvement of oxidative stress in MM, which is in line with our study.
Conclusion
These findings indicate the decreased antioxidant capacity and increased oxidative stress in patients with stage I MM. Oxidative stress could reduce the antioxidant activity of PON1 and ARE in such patients. Further studies are needed to clarify the possible mechanism(s) underlying the decreased enzyme activities.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflicts of interest The authors have declared no conflict of interest.
Ethics approval Study was approved by Ethical Committee of Urmia Medical University.
Acknowledgments
We thank the Urmia Medical University (Department of Medicine) for kindly supporting this project.
References
- Barlogie B, Epstein J. Multiple myeloma: biology and therapy. J Cancer Res Clin Oncol 1990;116(1):109–11. doi: 10.1007/BF01612651
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res 2011;183:25–35. doi: 10.1007/978-3-540-85772-3_2
- Kuku I, Aydogdu I, Bayraktar N, Kaya E, Akyol O, Erkurt MA. Oxidant/antioxidant parameters and their relationship with medical treatment in multiple myeloma. Cell Biochem Funct 2005;23(1):47–50. doi: 10.1002/cbf.1127
- Gerber M, Astre C, Segala C, Saintot M, Scali J, Simony-Lafontaine J, et al. Oxidant-antioxidant status alterations in cancer patients: relationship to tumor progression. J Nutr 1996;126(4):1201S–7S.
- Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev 2012;12(1):37.90–6.
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts II, LJ. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA 1990;87(23):9383–7. doi: 10.1073/pnas.87.23.9383
- Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012;2012:137289.
- Mackness MI, Mackness B, Durrington PN, Connelly PW, Hegele RA. Paraoxonase: biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol 1996;7(2):69–76. doi: 10.1097/00041433-199604000-00004
- Akcay MN, Yilmaz I, Polat MF, Akcay G. Serum paraoxonase levels in gastric cancer. Hepatogastroenterology 2003;50(2):cclxxiii–cclxxv.
- Elkiran ET, Mar N, Aygen B, Gursu F, Karaoglu A, Koca S. Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 2007;7:48. doi: 10.1186/1471-2407-7-48
- Krzystek-Korpacka M, Boehm D, Matusiewicz M, Diakowska D, Grabowski K, Gamian A. Paraoxonase 1 (PON1) status in gastroesophageal malignancies and associated paraneoplastic syndromes – connection with inflammation. Clin Biochem 2008;41(10–11):804–11. doi: 10.1016/j.clinbiochem.2008.03.012
- Stevens VL, Rodriguez C, Pavluck AL, Thun MJ, Calle EE. Association of polymorphisms in the paraoxonase 1 gene with breast cancer incidence in the CPS-II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2006;15(6):1226–8. doi: 10.1158/1055-9965.EPI-05-0930
- Balci H, Genc H, Papila C, Can G, Papila B, Yanardag H, et al. Serum lipid hydroperoxide levels and paraoxonase activity in patients with lung, breast, and colorectal cancer. J Clin Lab Anal 2012;26(3):155–60. doi: 10.1002/jcla.21503
- Caers J, Van Valckenborgh E, Menu E, Van Camp B, Vanderkerken K. Unraveling the biology of multiple myeloma disease: cancer stem cells, acquired intracellular changes and interactions with the surrounding micro-environment. Bull Cancer 2008;95(3):301–13.
- Taverne YJ, Bogers AJ, Duncker DJ, Merkus D. Reactive oxygen species and the cardiovascular system. Oxid Med Cell Longev 2013;2013:862423. doi: 10.1155/2013/862423
- Stojnev S, Ristic-Petrovic A, Jankovic-Velickovic L. Reactive oxygen species, apoptosis and cancer. Vojnosanit Pregl 2013;70(7):675–8. doi: 10.2298/VSP1307675S
- Martins Chaves M, Rocha-Vieira E, Pereira dos Reis A, de Lima e Silva R, Gerzstein NC, Nogueira-Machado JA. Increase of reactive oxygen (ROS) and nitrogen (RNS) species generated by phagocyting granulocytes related to age. Mech Ageing Dev 2000;119(1–2):1–8. doi: 10.1016/S0047-6374(00)00153-6
- Montuschi P, Barnes PJ, Roberts 2nd, LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J 2004;18(15):1791–800. doi: 10.1096/fj.04-2330rev
- Khadem-Ansari MH, Shahsavari Z, Rasmi Y, Mahmoodlo R. Elevated levels of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in esophageal squamous cell carcinoma. J Carcinog 2011;10:14. doi: 10.4103/1477-3163.79683
- Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI, et al. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer 2009;64(2):225–19. doi: 10.1016/j.lungcan.2008.08.015
- Zima T, Spicka I, Stipek S, Crkovska J, Platenik J, Merta M, et al. Antioxidant enzymes and lipid peroxidation in patients with multiple myeloma. Neoplasma 1996;43(2):69–73.
- Sharma A, Tripathi M, Satyam A, Kumar L. Study of antioxidant levels in patients with multiple myeloma. Leuk Lymph 2009;50(5):809–15. doi: 10.1080/10428190902802323
- Lodh M, Goswami B, Gupta N, Patra SK, Saxena A. Assessment of oxidative stress and inflammatory process in patients of multiple myeloma. Indian J Clin Biochem 2012;27(4):410–3. doi: 10.1007/s12291-012-0222-y
- Gangemi S, Allegra A, Alonci A, Cristani M, Russo S, Speciale A, et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm Res 2012;61(10):1063.7. doi: 10.1007/s00011-012-0498-7
- Ellidag HY, Eren E, Aydin O, Yildirim M, Sezer C, Yilmaz N. Multiple myeloma: relationship to antioxidant esterases. Med Princ Pract 2014;23(1):18–23. doi: 10.1159/000355826
- Sehitogullari A, Aslan M, Sayir F, Kahraman A, Demir H. Serum paraoxonase-1 enzyme activities and oxidative stress levels in patients with esophageal squamous cell carcinoma. Redox Rep 2014;19(5):199–205. doi: 10.1179/1351000214Y.0000000091
- Akcay MN, Polat MF, Yilmaz I, Akcay G. Serum paraoxonase levels in pancreatic cancer. Hepatogastroenterology 2003;50(2):ccxxv–ii.
- Siemianowicz K, Gminski J, Stajszczyk M, Wojakowski W, Goss M, Machalski M, et al. Serum HDL cholesterol concentration in patients with squamous cell and small cell lung cancer. Int J Mol Med 2000;6(3):307–11.
- von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs 2005;9(2):S35–8. doi: 10.1016/j.ejon.2005.09.001
- Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J 1997;11(6):457–65.

