Abstract
Objective
Dimethyl sulfoxide (DMSO) can damage hematopoietic progenitor cells (HPCs) at room temperature. To minimize the time of contact between DMSO and the thawed umbilical cord blood (UCB), there is an incentive to infuse the UCB as quickly as possible. However, the infusion of cryopreserved UCB also results in side effects. Currently, it is difficult to determine the optimal length of time for cryopreserved UCB infusion, not only to ensure the maximum effect of engraftment but also to reduce the toxicity of the cord blood infusion.
Methods
Ten units of cord blood were thawed to assess viability, apoptotic events of CD34+ cells and CD45+ cells, and the number of colony-forming units (CFUs) at four time points: 0, 10, 20, and 30 minutes post-thaw (PT). The infusion time, side effects, and the speed of platelet and neutrophil engraftment of the 10 patients were monitored.
Results
Within 30 minutes, the viability decreased (P < 0.01), the percentage of early apoptotic CD34+ cells and CD45+ cells was unchanged. At time point PT30, the number of CFUs was decreased compared to PT0 (P < 0.05), but it was unchanged within 20 minutes. All the 10 UCB cells engrafted well in patients.
Conclusion
This study indicates that post-thawed UCB HPCs are preserved for less than 30 minutes at room temperature; thus, the optimal length of time of cryopreserved cord blood infusion should be no more than 20 minutes after thawing.
Introduction
Currently, umbilical cord blood (UCB) is being developed as an alternative source of hematopoietic progenitor cells (HPCs) in unrelated allogeneic transplantation.Citation1 The limited cell dose is a significant obstacle to a larger utilization of UCB transplants, particularly for adult patients.Citation2 UCB cells are usually cryopreserved in dimethyl sulfoxide (DMSO) and frozen in liquid nitrogen until thawing and transfusion; however, at room temperature, DMSO can damage the HPCs.Citation3 Thus, cryopreserved UCB requires quick thawing at the bedside and immediate infusion over 5–15 minutes without any manipulation.Citation4,Citation5 However, several side effects ranging from mild to moderate have been described during UCB infusion, with the most frequent symptoms being hypertension, bradycardia, headache, nausea, vomiting, chest tightness, and tingling sensation in the fingers or abdomen.Citation4,Citation6 Life-threatening and even fatal cardiac toxicities have also been reported, such as arrhythmia, acute pulmonary edema, and coronary artery spasm.Citation7–Citation9 DMSO has been classically related to clinical toxicity during the infusion of cryopreserved graftsCitation4,Citation7 and can cause histamine release.Citation10 Animal studies have shown that these events are associated with cardiac toxicity and ischemic stroke.Citation11,Citation12 Moreover, rapid infusion will result in more DMSO infused into the patient's body per minute, which further aggravates the infusion reactions. Additionally, studies have demonstrated that the volume/kg infused and actual time of infusion are important variables related to cardiovascular toxicities.Citation13
UCB products have traditionally been thawed using a washing method (diluted and then centrifuged) intended to reduce DMSO toxicity and cell lysis; however, this process can result in cell loss.Citation14 Regan et al.Citation15 have shown that dilution with albumin and dextran could increase cell viability compared to the thaw-only, moreover, there was no significant difference in the either the frequency or the severity of adverse events during cord blood infusion. However, in China, this method is not employed because is considered too expensive. In our center, the cord blood (approximately 30 ml of UCB units) is infused at the bedside immediately after thawing for 10–15 minutes, causing a wide variety of side effects, such as hypertension, headache, chest discomfort, and other toxicities. Hence, post-thaw UCB is supposed to be infused in a longer period of time to reduce DMSO toxic effects on patients.
To test the optimal length of time of post-thaw UCB infusion, the in vitro effect of time on UCB products at room temperature by evaluating cell viability, early apoptosis of CD34+ cells and CD45+ cells, and the number of colony-forming units (CFUs) was designed.
Materials and methods
Collection, processing, and cryopreservation of cord blood units
The 10 UCB units in this study were obtained from the Shanghai (n = 5), Beijing (n = 2), Guangzhou (n = 1), Tianjin (n = 1), and Shandong (n = 1) UCB banks. Three UCB units were stored for 1 year, two units for 2 years, three units for 3 years, one unit for 5 years, and one unit for 10 years. The methods of collection, processing, and cryopreservation are similar between the UCB banks. UCB units were collected from eligible mothers with placenta in utero by draining the cord blood via gravity into a bag containing an anticoagulant citrate phosphate dextrose solution. After collection, the bag was sent to the processing facility within 24 hours. The UCB product was then mixed with 6% hetastarch in saline (Hespan; B. Braun Medical Inc., Irvine, CA, USA) at a 1:4 ratio, inverted at 10°C for 30 minutes, and centrifuged at 50g for 6 minutes. The packed red cells were drained, and the nucleated cell-rich plasma was subjected to centrifugation at 400g for 13 minutes. Ice-cold freezing medium containing equal volumes of DMSO (Edwards Lifesciences Inc., Bedford, OH, USA) and dextran 40 (B. Braun Medical Inc.) was added to the remaining product at a 1:4 ratio in a 50-ml Cryocyte bag (Baxter Inc., Irvine, CA, USA), yielding a final concentration of 10% DMSO. The cryopreservation protocol was performed as follows: −5°C/minute cooling to 4°C, −1°C/minute cooling to −45°C, and −5°C/minute cooling to −100°C. After freezing, the cells were transferred into liquid-phase nitrogen for long-term storage.Citation16
Thawing of cord blood units
Units were removed from liquid nitrogen storage and thawed in a 40°C water bath. Once thawed, 0.2 ml of UCB was removed after the informed consent of the patients was obtained. The 0.2 ml of UCB was divided into four fractions, then maintained at room temperature and diluted with 4 volumes of albumin– dextran solution (5% human serum albumin and 10% dextran 40),Citation17 respectively, at the following prescribed time: 0 (the time point at UCB absolutely thawing), 10, 20, and 30 minutes after UCB thawing. At the same time, the viability, apoptotic events of CD34+ cells and CD45+ cells, and the number of CFUs were evaluated.
Measurement of viability
Cell viability was determined by Trypan blue dye exclusion to distinguish between live and dead cells.
Annexin V assessment method of CD34+ and CD45+ cell enumeration
The cells were resuspended in 1× Annexin V (Ann V)-binding buffer at a concentration of 1 × 106 cells/ml. Approximately 100 µl of the solution (1 × 105 cells) was transferred to a 5-ml culture tube and labeled with anti-CD34-APC (BD Biosciences, San Jose, CA, USA) and anti-CD45-Percp-Cy5.5 (BD Biosciences, San Jose, CA, USA), as well as Ann V-fluorescein isothiocyanate (BD Biosciences, San Jose, CA, USA) and propidium iodide (PI) (BD Biosciences, San Jose, CA, USA) for 20 minutes at room temperature in the dark. After labeling, 400 µl of 1× binding buffer was added to each tube, and the samples were analyzed by flow cytometry (FACSCantoTMII, BD Biosciences, San Jose, CA, USA) within 1 hour. This method was used to set the control gate with the standard enumeration to calculate the percentage of PI(−) cells.
CFU assays
The formation of CFUs was carried out in a methylcellulose culture medium containing a combination of growth factors (MethoCult 4434, Stem Cell Technologies, Vancouver, Canada). Duplicate 1-ml cultures, each containing 1 × 105 cells, were plated in 35-mm culture dishes and cultured at 37°C in a humidified 5% CO2 incubator for 14 days. A colony was defined as an aggregate of more than 40 cells and was counted using an inverted microscope. The number of CFUs was reported as total colonies per component.
Assessment of infusion-related toxicities and engraftment
Breathing rate, pulse, blood pressure, and oxygen saturation of patients were monitored starting before infusion until 2 hours after UCBT, recorded every 5 minutes during infusion, every 30 minutes for 2 hours after infusion. Clinical symptoms were also recorded, according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3. Time to neutrophil engraftment was defined as the time required to reach the first of three consecutive days with an absolute neutrophil count of ≥0.5 × 109/l. Platelet (PLT) engraftment, in contrast, was considered as the first of 7 consecutive days at an absolute PLT count >20 × 109/l without transfusional support.
Statistical analysis
The results are shown as the means ± SD. Significant differences over time in the group were analyzed by analysis of variance. The paired t-test was used to compare the pre-freezing cell number with its post-thaw counterpart. SPSS version 10.0 was used for all statistical analyses, and a value of P < 0.05 was considered to be statistically significant.
Results
Viability of UCB cells after thawing
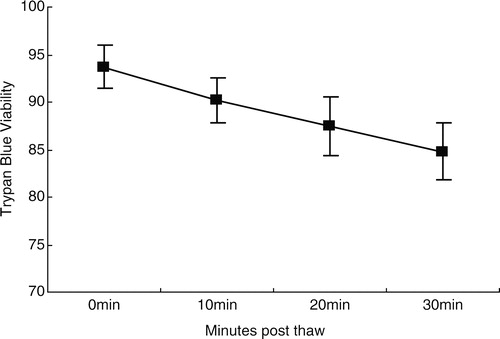
As assessed by Trypan blue staining, viability was significantly different at time points 0, 10, 20, and 30 minutes post-thaw (PT) (). Cell viability decreased over time, from 93.7 ± 2.21% at PT0 to 90.2 ± 2.35% at PT10, 87.5 ± 3.06% at PT20, and 84.8 ± 2.94% at PT30 (P < 0.01 for all time points).
The CD34+ PI− and CD45+ PI− gates contain early apoptotic cells
Ann V(+) binding to CD34+ PI(−) cells and CD45+ PI(−) cells in UCB samples was observed (). The percentage of Ann V(+) binding to CD34+ PI(−) cells was 11.61 ± 2.16% at PT0; in contrast, the percentage of Ann V(+) binding to CD45+ PI(−) cells was 16.09 ± 4.28% at PT0. However, the number of early apoptotic CD34+ and CD45+ PI(−) cells was similar over time, and the differences were not significant (P > 0.05 for both cell markers, ).
Figure 2. Flow cytometric analysis of apoptotic marker expression in post-thaw UCB samples. The gating of CD34+ cells (P2) (A) and CD45+ cells (P1) (B) from UCB samples after thawing. Gated CD34+ cells (P2) at time PT0 (C), 10 (D), 20 (E), and 30 minutes (F) were analyzed for apoptotic markers as assessed by staining with Ann V and PI. Gated CD45+ cells (P1) from UCB samples at time PT0 (G), 10 (H), 20 (I), and 30 minutes (J) were also analyzed for apoptotic markers.
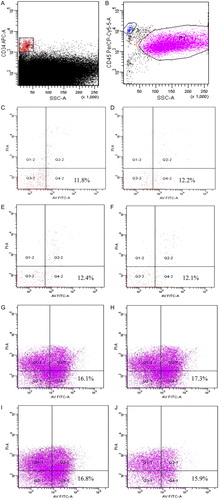
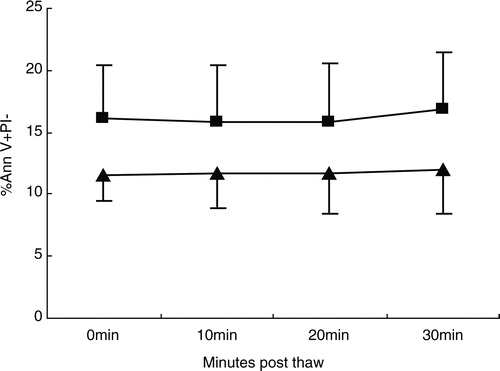
Figure 3. The percentage of Ann V(+) binding to CD34+ PI(−) cells and CD45+ PI(−) cells in UCB samples (n = 10). The number of early apoptotic CD34+ PI(−) cells and CD45+PI(−) cells was similar over time, and the differences were not significant (P > 0.05 for both cell markers). (▴) Ann V(+)PI(−) CD34+ cells; (▪) Ann V(+)PI(−)CD45+ cells.
CFU recovery
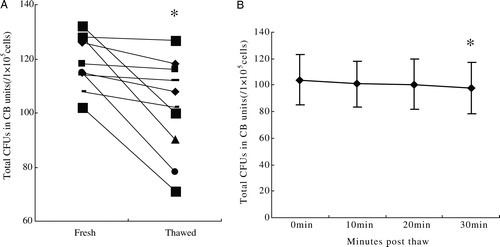
The ability to form CFUs (relative to prefreeze content) was 85.2 ± 13.09% after thawing, which demonstrated a significant decrease (P = 0.01, A). The number of CFUs at time point PT30 was decreased in comparison to PT0 (P < 0.05), but the number of CFUs was unchanged within 20 minutes (P > 0.05, B).
Figure 4. (A) The ability to form CFUs (relative to prefreeze content) was 85.2 ± 13.09% after thawing at PT0, which demonstrated a significant decrease (P = 0.01). (B) The number of CFUs was 104 ± 18.8, 101 ± 17.5, 101 ± 18.8, and 98 ± 19.3, respectively, at PT0, 10, 20, and PT30, at time point PT30, the number of CFUs was decreased in comparison to PT0 (P < 0.05), but the number of CFUs was unchanged within 20 minutes (P > 0.05).
Infusion-related toxicities and engraftment
The main patient characteristics are shown in . In the study, 90% of patients experienced toxicities related to infusion of UCB units, range from mild to moderate, and none life-threatening toxicity was reported (). All the 10 patients recovered hematopoiesis at a median time of 19 days (range 14–31) for neutrophils and 57 days (range 47–72) for platelets.
Table 1. Characteristics of patients
Table 2. Toxicity associated with 10 UCB infusions
Discussion
Traditionally, cord blood units have been infused intravenously over 5–15 minutes but also have been associated with a variety of adverse reactions. Determining an optimal length of time for post-thaw UCB infusion is an urgent issue to address not only to decrease the time-dependent loss of cell viability but also to reduce the DMSO toxicity of cord blood infusion.
Cell viability is an important characteristic when assessing UCB units, with Trypan blue staining being used at most centers. In this study, we observed that cell viability decreased over time, which was consistent with other reports.Citation15,Citation18 However, at time point PT30, more than 80% of the cells were still viable, suggesting that keeping the thawed cord blood at room temperature for 30 minutes resulted in only a few dead cells. Additionally, cell death is primarily due to the loss of differentiated cell populations, such as mature granulocytes, which are very sensitive to cryopreservation and are mainly positively stained after thawing. However, these cells have no impact on hematopoietic engraftment.Citation19 In the study, the median duration of UCB infusion was 10 minutes, and all the 10 patients recovered hematopoiesis, thus, there was statistical significance in cell viability, but the cell death did not show any real biological significance on engraftment.
Apoptosis is a common cell death pathway, which is initiated by a variety of different stimuli. In apoptotic cells, the membrane phospholipid phosphatidylserine (PS) is translocated from the inner leaflet to the outer leaflet of the plasma membrane, resulting in the exposure of PS to the external cellular environment.Citation20 The protein Ann V has a high affinity for PS and binds to cells with exposed PS. During the initial stages of apoptosis, the cell membrane remains intact, whereas in the late apoptotic/necrotic stages, the cell membrane loses its integrity and becomes porous. The fluorescent DNA binding agent PI enters late apoptotic/necrotic cells but not early apoptotic cells, which still possess an intact cell membrane. The use of Ann V binding with PI staining can thus distinguish between early apoptotic cells and late apoptotic/necrotic cells.
Transplant data have indicated that the percentage of CD34+ cells infused is an important predictor of the UCB transplantation outcome.Citation21–Citation23 However, UCB units contain significant percentages of early apoptotic CD34+ cells after thawing, which can influence the outcome of UCB transplantation.Citation21 Duggleby et al.Citation24 noted that the assessment of apoptotic CD34+ cells by Ann V labeling can improve the prediction of the number of CFUs, thus providing an assay to rapidly test the potency of UCB units for clinical use. The percentage of early apoptotic CD34+ cells at PT0 was 11.61 ± 2.17% in this study; over time, the differences among of the four time points were not significant (P > 0.05). Our observations corroborate other reports that early apoptotic CD34+ cells are induced by cryopreservation and thawing,Citation25,Citation26 and the remaining functional CD34+ cells can maintain their cloning capacity over time.
The assessment of CFUs is the most stringent test of hematopoietic potential and minimizes the risk of engraftment failure.Citation27 Currently, the CFU dose has been recognized as one of the best predictive markers of UCB transplantation success.Citation28 This study yielded a CFU recovery of 85.2% at PT0, which was in agreement with another published study. Alonso et al.Citation29 analyzed 4055 UCB units and found a median CFU yield of 84%; of these analyzed units, 25 were used for transplantation and showed CFU yields of 76%. In contrast, the percentage of Ann V(+) and PI(−) CD34+ cells after thawing suggests that the use of Ann V labeling to assess viable cell loss mimicked the observed decrease in CFUs. In this study, there was no change in the CFU yield within 20 minutes, but the yield was decreased at PT30 compared to PT0 (P < 0.05). We suspect that the passage of time after thawing resulted in cell loss, and the presence of dead cells and cell debris in the UCB units had a negative impact on the cultures. Additionally, DMSO may be another factor that has an effect on the ability to form colonies.Citation30 Douay et al.Citation3 reported that cells maintained in DMSO at 4°C for a short time period demonstrated a substantial loss of granulocyte-macrophage precursor recovery.
In conclusion, this study indicates that post-thaw UCB HPCs are preserved for less than 30 minutes at room temperature, and less than 20 minutes may be the optimal length of time for the infusion of post-thaw cord blood. However, this study is limited in size, and as we only observed the in vitro effect on cell content 30 minutes after thawing, the effect on cell content at later time points is still unknown. Therefore, more time points and in vivo effect on the cell content must be collected to determine if less than 20 minutes post-thaw is the optimal length of time for cord blood infusion.
Acknowledgements
We would like to thank the patients undergoing hematopoietic stem cell transplantation for donating cord blood to our study, without their contribution, our study cannot be completed. We are also grateful to Professor Li Qing for his excellent technical support.
Funding
This work was supported in part by grants from Anhui Provincial Health Bureau on 2012, China (No. 12010402120).
References
- Gluckman E. Milestones in umbilical cord blood transplantation. Blood Rev. 2011;25(6):255–9.
- Dalle JH, Duval M, Moghrabi A, Wagner E, Vachon MF, Barrette S, et al. Results of unrelated transplant search strategy using partially HLA-mismatched cord blood as an immediate alternative to HLA-matched bone marrow. Bone Marrow Transplant. 2004;33:605–11.
- Douay L, Gorin NC, David R, Stachowiak J, Salmon C, Najman A, et al. Study of granulocyte-macrophage progenitor (CFUc) preservation after slow freezing of bone marrow in the gas phase of liquid nitrogen. Exp Hematol. 1982;10:360–6.
- Konuma T, Ooi J, Takahashi S, Tomonari A, Tsukada N, Kobayashi T, et al. Cardiovascular toxicity of cryopreserved cord blood cell infusion. Bone Marrow Transplant. 2008;41:861–5.
- Petterson TE, Gabriel M, Tiedemann K, Teague L, Shaw PJ, Baker D, et al. Outcome following unrelated cord blood transplant in 136 patients with malignant and non-malignant diseases: a report from the Australian and New Zealand children's haematology and oncology group. Bone Marrow Transplant. 2009;43:207–15.
- Hahn T, Bunworasate U, George MC, Bir AS, Chinratanalab W, Alam AR, et al. Use of nonvolume-reduced (unmanipulated after thawing) umbilical cord blood stem cells for allogeneic transplantation results in safe engraftment. Bone Marrow Transplant. 2003;32:145–50.
- Ruiz-Delgado GJ, Mancías-Guerra C, Tamez-Gómez EL, Rodríguez-Romo LN, López-Otero A, Hernández-Arizpe A, et al. Dimethyl sulfoxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol. 2009;122:1–5.
- Ma RW, Kwan JM, Ma DD, Fay KC. Acute life-threatening cardiovascular toxicity with umbilical cord blood infusion: the role of dextran. Am J Hematol. 2010;85:722–4.
- Petropoulou AD, Bellochine R, Norol F, Marie JP, Rio B. Coronary artery spasm after infusion of cryopreserved cord blood cells. Bone Marrow Transplant. 2007;40:397–8.
- KLIGMAN AM. Topical pharmacology and toxicology of dimethylsulfoxide.1. JAMA. 1965;193:796–804.
- Chaloupka JC, Huddle DC, Alderman J, Fink S, Hammond R, Vinters HV. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete embolization model. AJNR Am J Neuroradiol. 1999;20:401–10.
- Shlafer M, Karow AM Jr. Pharmacological effects of dimethyl sulfoxide on the mammalian myocardium. Ann N Y Acad Sci. 1975;243:110–21.
- Milone G, Mercurio S, Strano A, Leotta S, Pinto V, Battiato K, et al. Adverse events after infusions of cryopreserved hematopoietic stem cells depend on non-mononuclear cells in the infused suspension and patient age. Cytotherapy. 2007;9:348–55.
- Beaujean F, Hartmann O, Kuentz M, Le Forestier C, Divine M, Duedari N. A simple, efficient washing procedure for cryopreserved human hematopoietic stem cells prior to reinfusion. Bone Marrow Transplant. 1991;8:291–4.
- Regan DM, Wofford JD, Wall DA. Comparison of cord blood thawing methods on cell recovery, potency, and infusion. Transfusion 2010;50:2670–5.
- Wu JY, Liao C, Xu ZP, Chen JS, Gu SL, Huang YN, et al. Banking and transplantation of umbilical cord blood in Guangzhou, China. Cytotherapy. 2006;8:488–97.
- Liu J, Zhou SY, Huang GD, Huang Y, Wang YS, Chen DZ, et al. Cryobiological characteristics of placental cord blood preserved in BioArchive auto-preserved liquid nitrogen system. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002;10:261–4.
- Hayakawa J, Joyal EG, Gildner JF, Washington KN, Phang OA, Uchida N, et al. 5% Dimethyl sulfoxide (DMSO) and pentastarch improves cryopreservation of cord blood cells over 10% DMSO. Transfusion 2010;50:2158–66.
- Picardi A, Arcese W. Quality assessment of cord blood units selected for unrelated transplantation: a transplant center perspective. Transfus Apheresis Sci. 2010;42:289–97.
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16.
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8.
- Allan DS, Keeney M, Howson-Jan K, Popma J, Weir K, Bhatia M, et al. Number of viable CD34+ cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:967–72.
- Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collection in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9.
- Duggleby RC, Querol S, Davy RC, Fry LJ, Gibson DA, Horton RB, et al. Flow cytometry assessment of apoptotic CD34+cells by annexin V labeling may improve prediction of cord blood potency for engraftment. Transfusion 2012;52:549–59.
- de Boer F, Dräger AM, Pinedo HM, Kessler FL, Monnee-van Muijen M, Weijers G, et al. Early apoptosis largely accounts for functional impairment of CD34+ cells in frozen-thawed stem cell grafts. J Hematother Stem Cell Res. 2002;11:951–63.
- Sparrow RL, Komodromou H, Tippett E, Georgakopoulos T, Xu W. Apoptotic lymphocytes and CD34+ cells in cryopreserved cord blood detected by the fluorescent vital dye SYTO 16 and correlation with loss of L-selectin (CD62L) expression. Bone Marrow Transplant. 2006;38:61–7.
- Iori AP, Cerretti R, De Felice L, Screnci M, Mengarelli A, Romano A, et al. Pre-transplant prognostic factors for patients with high-risk leukemia undergoing an unrelated cord blood transplantation. Bone Marrow Transplant. 2004;33:1097–105.
- Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K, et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–89.
- Alonso JM III, Regan DM, Johnson CE, Oliver DA, Fegan R, Lasky LC, et al. A simple and reliable procedure for cord blood banking, processing, and freezing: St Louis and Ohio Cord Blood Bank experiences. Cytotherapy. 2001;3:429–33.
- Goldman JM, Th'ng KH, Park DS, Spiers AS, Lowenthal RM, Ruutu T. Collection, cryopreservation and subsequent viability of haemopoietic stem cells intended for treatment of chronic granulocytic leukaemia in blast-cell transformation. Br J Haematol 1978;40:185–95.