Abstract
Objectives: Whether a relationship between fish consumption and non-Hodgkin lymphoma (NHL) risk exists is an open issue. We carried out a meta-analysis to explore this association according to the published observational studies.
Methods: We performed a search of databases in MEDLINE and EMBASE to identify relevant studies. We derived meta-analytic estimates using random-effects models, and assessed between-study heterogeneity using the Cochran's Q and I2 statistics.
Results: We identified a total of seven case–control and two prospective cohort studies, including 7696 subjects with NHL. The summary relative risks (SRRs) estimated for NHL were 0.80 (95% confidence intervals (CIs): 0.68–0.94) for those in the highest fish consumption category compared with those in the lowest consumption category. There was evidence of significant heterogeneity across studies (Q = 26.72, Pheterogeneity = 0.002, I2 = 66.3%). Stratified analysis by study design indicated that a significant risk association between fish consumption and NHL was observed in case–control studies, but not in cohort studies. Based on the dose–response meta-analysis, the SRRs of NHL were 0.85 (95% CIs: 0.71–1.01) for three servings increased per week of fish consumed with evidence of significant heterogeneity (Pheterogeneity = 0.007, I2 = 63.9%).
Conclusions: Findings from our meta-analysis indicate that consumption of fish may be not related to NHL risk.
Introduction
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of malignancies arising from the lymphoid tissue of immune system, which ranks the seventh most commonly malignant tumor among men and women in the USA.Citation1 Temporal shifts and geographic variations in the incidence of NHL point to possible involvement of environmental factors in lymphomagenesis. In addition to immune dysregulation, virus infection, certain medical conditions,Citation2 and several lifestyle factors, such as tobacco smokingCitation3 and obesity,Citation4 are implicated to play a potential role in the etiology of this disease. Since the actions of dietary factors in immune system function have been reported, the development of NHL has also been linked to some dietary factors, such as intake of processed meat, poultry, and other animal products, although the evidence is both inconsistent and limited.Citation5–Citation7
Consumption of fish is part of the usual diet of most people worldwide, which is suggested to play a role in the prevention of several cancers.Citation8,Citation9 It was reported that fish intake may lead to reduced colorectal cancer risk,Citation9 although it may not be associated with risk of pancreatic cancerCitation8 and esophageal cancer.Citation10 The n-3 fatty acids rich in fish are important components of cell membranes,Citation11 and play a role in suppressing mutations, enhanced cell apoptosis and cell growth inhibition, thus reducing the carcinogenesis.Citation12,Citation13
To date, several epidemiological studies have focused on questions of whether intake of fish is related to NHL risk. Although one study reported a significant protective association with NHL risk,Citation14 others show a non-significant association for fish consumption.Citation6,Citation15–Citation21 Interestingly, an analysis of Canadian National Enhanced Cancer Surveillance System data, which examined the risk of NHL in animal-related occupations, observed an unexpected finding that there was a substantial reduction in the risk of NHL among those in fishing occupations (odds ratio = 0.6).Citation22 Researchers hypothesized that this inverse association may be due to increased dietary fish intake in people who work in fish-related occupations.Citation22
No quantitative reviews were available to date for the development of NHL with consumption of fish. We therefore preformed the meta-analysis to assess this association following the guidelines of meta-analysis of observational studies in epidemiology.Citation23
Methods
Data sources and searches
Studies were included if they were published in English-language journals up to 30 June 2014 and reported the association between fish consumption and the incidence of NHL. Two independent researchers (Y.L. and S.W.Y.) performed a search of databases MEDLINE and EMBASE using the following text word: (1) cancer OR lymphoma, (2) seafood OR fish OR shellfish, (3) case–control OR cohort. Additional studies were retrieved by reviewing the reference lists of the relevant articles.
Study selection
Articles were included according to following criteria: (1) studies were published as an observational study using a case–control or cohort design, and (2) studies presented rate ratios/odds ratios and their corresponding 95% confidence intervals (CIs) for risk of NHL relating to fish intake with the lowest category as the reference. Two of us (Y.L. and S.W.Y.) independently assessed titles and abstracts of potentially eligible studies. Discrepancies were resolved by discussion. We excluded experimental and mechanistic studies, non-peer-reviewed articles, ecologic assessments, and correlation studies. If separate reports from the same study were published, the ones with larger cases were included.
Data extraction
From the included studies, two of us (Y.L. and S.W.Y.) extracted the information independently. Disagreements between reviewers were resolved by consensus. We included the following data: the first author's last name, locations, design, publication year, case and comparator group size, dietary questionnaire, adjusted confounders, and odds ratio or risk ratio estimates with corresponding 95% CIs for the highest vs. lowest intake level. Only the relative risk (RR) estimates for fresh fish were extracted, in case that studies presented data on fresh and other types of fish, respectively.Citation14,Citation16,Citation20 We counted one studyCitation19 as two separate one, which reported the results according to t(14;18) status, one of the most common chromosomal abnormalities in NHL.Citation19
Statistical methods
We estimated the weighted RRs (95% CIs) of NHL for fish consumption using the inverse variance method under a random effect model.Citation24 We calculated summary relative risk (SRR) and 95% CI to measure the impact of the highest level of fish consumption on the risk of NHL.
Assessment of heterogeneity was performed using both Cochran's Q (testing the heterogeneity among studies) and ICitation2 (determining the degree of inconsistency across studies). For Q statistics, heterogeneity exists when a P value was less than 0.1. ICitation2 value over 50% indicates that substantial heterogeneity may be present, and less than 25% is considered to have no significant heterogeneity.Citation25 We carried out strata and linear meta-regression analysis based on geographic locations (Europe, America, and Asia), study design (case–control vs. cohort study), and type of food frequency questionnaires (FFQ, validated vs. not validated). Confounders were defined as smoking status, body mass index (BMI), alcohol use, and dietary energy intake. We also examined the associations for subtypes of NHL (diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL)) because of their different etiopathogenesis.
We then conducted sensitivity analyses to test the robustness of our findings: whether the results would be markedly affected by a single study, whether repeated analysis by a fixed-effects model would generate similar results.
When performing linear dose–response analysis, we calculated the RR per three servings per week increase of fish consumption. We used generalized least-squares trend estimation analysis or variance-weighted least squares regression analysis for trend estimation.Citation26,Citation27 For two studies that reported results for fish intake in grams/1000 kcal/day,Citation6,Citation21 we derived servings using the average energy intake reported in the articles and by assuming that one serving equals 100 g. Means or medians of the intake categories were used when reported in the articles. In case medians were unavailable, we used the average intake level in each category. Zero consumption was used as boundary when the lowest category was open ended and if the highest category was open ended, we calculated the open-ended boundary using an interval length of the width of the closest interval.
Publication bias was assessed by visual inspection of asymmetry in funnel plots and the further Begg's adjusted rank correlation and Egger's regression test.Citation28,Citation29 To reduce the potential influence of publication bias, we used the trim-and-fill method.Citation30 STATA, version 11.0 was used for all statistical analyses (STATA, College Station, TX, USA), and a two-sided P values <0.05 were considered to be statistically significant.
Results
Search results and study characteristics
The detailed steps of our literature search are shown in supplementary Figure (all supplementary material is available online at http://www.maneyonline.com/doi/suppl/10.1179/1607845414Y.0000000215). In brief, our search found a total of 3017 publications according to the search strategy, of which 45 were considered of potential value and the full text was retrieved for detailed evaluation. From the reference review, we included additional five articles. Thirty-two non-relevant publications were subsequently excluded from the meta-analysis, four were excluded because they did not report the RR estimates and/or corresponding 95% CIs, or sufficient information to calculate them. Five articles were excluded since they reported the same studies. Thus, the present analyses were based on nine studies (Table ): two prospective cohort,Citation6,Citation15 four population-based,Citation18–Citation21 and three hospital-based case–control studies.Citation14,Citation16,Citation17 A total of 7696 subjects with NHL are included. Six studies were from the USA; the remaining three studies were from Italy, Canada, and Japan, respectively. FFQ were utilized to evaluate dietary data on fish consumption in all studies. Only one studyCitation14 reported a significantly decreased risk of NHL with fresh fish intake; and the remaining eight studies presented non-significantly inverse relationships.
Overall analysis
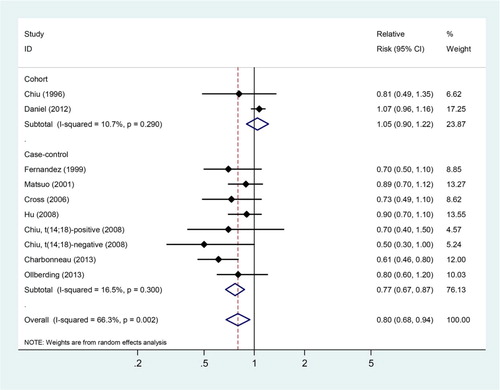
As shown in Fig. , according to a random-effects model, the SRRs estimated for NHL for the highest group compared with the lowest group of fish intake were statistically significant (SRRs = 0.80, 95% CIs: 0.68–0.94). There was evidence of strong heterogeneity across these studies (Q = 26.72, Pheterogeneity = 0.002, ICitation2 = 66.3%).
In stratified analysis of study design, we observed a lower RR of NHL in case–control studies (SRRs = 0.77, 95% CIs: 0.67–0.87; n = 7 studies), but not in cohort studies (SRRs = 1.05, 95% CIs: 0.90–1.22; n = 2 studies). Furthermore, subgroup analyses by source of control showed an inverse association in both hospital-based (SRRs = 0.73, 95% CIs: 0.57–0.94) and in population-based case–control studies (SRRs = 0.80, 95% CIs: 0.68–0.94).
Excluding two studies which were conducted in Japan and in Italy, respectively, the SRRs were still significant for studies conducted in America (SRRs = 0.79, 95% CIs: 0.64–0.97; Pheterogeneity = 0.001, ICitation2 = 71.2%). Whether or not adjusted by smoking status significantly altered the risk association between fish consumption and NHL risk (adjusted by smoking: SRRs = 0.93, 95% CIs: 0.76–1.15; not adjusted by smoking status: SRRs = 0.75, 95% CIs: 0.65–0.86; P for difference = 0.085). Whereas type of FFQ (validated vs. not validated) and whether or not adjusted by BMI, alcohol drinking and dietary energy intake did not significantly change the summary risk estimates (Table ).
Table 1 Characteristics of studies of fish consumption and risk of non-Hodgkin lymphoma
Table 2 Stratified meta-analyses of fish consumption and risk of non-Hodgkin lymphoma
We then conducted a meta-regression analysis to investigate the impact of the above study characteristics on the association between fish consumption and NHL risk. Both study design (P = 0.042) and confounders adjusted for smoking status (P = 0.085) were significant factors for the association between fish intake and NHL risk.
The sensitivity analysis revealed that none of the studies significantly influenced the summary risk estimates, with the pooled RRs ranging from 0.81 (95% CIs: 0.65–0.97) after excluding study from Hu et al.Citation31 to 0.88 (95% CIs: 0.76–0.99) after excluding study from Charbonneau et al.Citation14 When the analysis was repeated using a fixed-effects model, the results were essentially the same (data not shown).
NHL subtypes
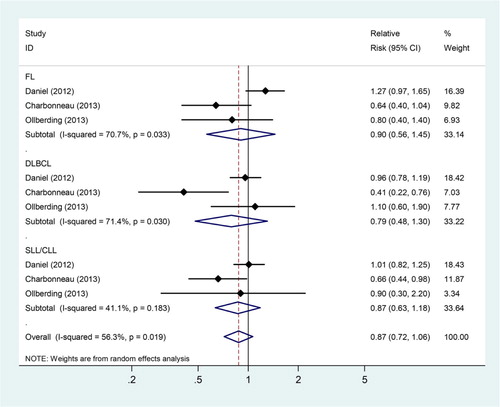
Three studiesCitation6,Citation14,Citation21 presented risk estimates for the association between fish consumption and NHL subtypes, respectively. We found that consumption of fish was not associated with the development of FL (SRRs = 0.90, 95% CIs: 0.56–1.45; Pheterogeneity = 0.033, ICitation2 = 70.7%), DLBCL (SRRs = 0.79, 95% CIs: 0.48–1.30; Pheterogeneity = 0.030, ICitation2 = 71.4%), and SLL/CLL (SRRs = 0.87, 95% CIs: 0.64–1.18; Pheterogeneity = 0.183, ICitation2 = 41.1%; Fig. ).
Publication bias
Egger's test revealed evidence of publication bias (P = 0.026), but Begg's test did not (P = 0.152; supplementary Figure ). The trim-and-fill method indicated that no additional risk estimate was needed to balance the funnel plot, and the summary risk estimates were not changed.
Dose–response analysis
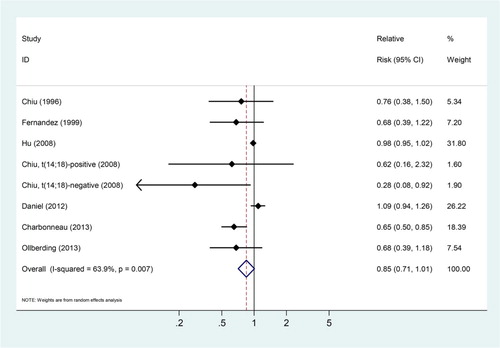
Seven studies could be used in the dose–response meta-analysis of fish consumption and risk of NHL.Citation6,Citation14–Citation16,Citation19–Citation21 The SRRs of NHL were 0.85 (95% CIs: 0.71–1.01) for three servings increased fish consumed per one week, with evidence of significant heterogeneity across these studies (Pheterogeneity = 0.007, ICitation2 = 63.9%; Fig. ).
Discussion
The important effects of dietary intake on cancer prevention have been received much attention recently.Citation10 The present meta-analysis showed a role of fish consumption in the lowering of NHL risk. Based on the highest vs. lowest analysis in a random-effects model, we found there was a 20% decreased risk of NHL (95% CIs: 0.68–0.94). Significant heterogeneity was observed among these studies, which may be partially ascribed to discrepancy in confounder adjusted by smoking status and study design. Dose–response meta-analysis showed that the SRRs of NHL were 0.85 (95% CIs: 0.71–1.01) per three servings per week increase in fish consumption.
Results from our meta-analysis observed that fish consumption was inversely associated with NHL risk in case–control studies (SRRs = 0.77, 95% CIs: 0.67–0.87), which drove the main findings, but not in cohort studies (SRRs = 1.05, 95% CIs: 0.90–1.22). Case–control design is more susceptible to recall biases and selection bias than cohort design. In fact, exposure information was obtained after NHL diagnosis in several case–control studies included in the analysis; these data may be subject to recall bias and inaccurate measurements of fish intake. Furthermore, it is more likely for cases to over report foods considered unhealthy and underreport foods considered healthy in an attempt to explain their illness. Thus, we should treat our results with caution because the overall findings of reduction in NHL risk should be overemphasized.
Individual studies generally had low statistical power to address the association between fish consumption and subtypes of NHL because information on this association is sparse. Thus, a major strength of this study was the opportunity to resume the available evidence and to provide separate summary estimates for several NHL subtypes. Accordingly, we found that the relation between fish consumption and NHL was not statistically significant for various cancer subtypes. We should note that our results may be only due to chance, because they were based on only three studies. More studies focusing on the association between NHL subtypes and fish consumption are needed.
Several potential mechanisms have been suggested to explain why fish intake may play a preventive role in the development of cancers. Fish is an ideal source of long-chain n-3 polyunsaturated fatty acids (PUFAs), which play a role in suppressing mutations, enhanced cell apoptosis and cell growth inhibition, thus reducing the carcinogenesis for various sites.Citation12,Citation13 Results from two case–control studies have shown that supplementing the diet with n-3 PUFAs was inversely associated with NHL risk.Citation14,Citation32 Furthermore, the preventive effects of dietary n-3 PUFAs on the development and progression of certain types of cancer have been observed in animal studies.Citation33,Citation34
The advantages of the current study include as following: (1) to our knowledge, this study is the first to quantitatively review the association between fish consumption and NHL risk; (2) study populations in the included studies were as homogeneous as possible because we used the comprehensive and extended exclusion criteria; (3) the ascertainment of NHL was from histological findings in most of the included studies, except two cohort studies.Citation6,Citation15
However, the current meta-analysis has several shortcomings. First, our findings were likely to be influenced by imprecise assessments of dietary fish intake, which could have led to overestimation of the range of intake and underestimation of the magnitude of the relationship between dietary intake and cancer risk.Citation35,Citation36 The ranges and units of fish intake varied considerably across the studies. For example, results according to servings per week, grams per day, and servings per month were reported in some studies, and quartiles of fish consumption without demarcating the cut-points were reported in the other study.Citation18 In addition, validated dietary questionnaires were not available in several studies.Citation16,Citation17,Citation19 However, subgroup analyses showed that use of a validated vs. non-validated FFQ did not significantly modify the relationship between dietary fish intake and NHL risk.
Second, severe heterogeneity is found across the included studies (Q = 26.72, Pheterogeneity = 0.002, ICitation2 = 66.3%), which may be partially explained by varying definitions of fish in different studies. Three of the nine studies provided data on consumption of fresh fish and other types of processed fish, but we selected only data on fresh fish consumption.Citation14,Citation16,Citation20 Although the association between processed fish intake and risk of NHL remains in conclusive, an enhanced risk of cancer associated with consumption of salted fish has been suggested in several studies.Citation37,Citation38 Compared with fresh-water fish, marine fish contain higher levels of n-3 and lower levels of n-6 fatty acids. It is reported that although n-3 fatty acids may protect against cancer development, n-6 fatty acids may not be the case.Citation33,Citation39 Smoked fish or processed foods are found to generate chemical carcinogens such as nitrites and their related compounds, or food mutagens such as heterocyclic amines, which may induce immunotoxicity,Citation40 and be associated with an increased risk of lymphoma in rodents.Citation41 However, we assessed only total fish consumption because most of these studies were not primarily designed to investigate the effect of fish consumption on NHL risk, and did not specify what type of fish was consumed.
Third, given that the recognized risk factors in the etiology of NHL remain largely unclear, residual confounders are always of concern in observational studies. In nutritional epidemiological studies, adjustment for total energy intake is important to account for potential confounding factors by dietary correlates.Citation42 A recent meta-analysis by Tramacere et al.Citation43 observed evidence of a favorable role of alcohol drinking on NHL risk, although lacking a biological explanation for this. Additionally, obesity is also suggested to be a risk factor for NHL.Citation4 In stratified analyses according to studies whether or not adjusted by these potential confounders, there was no significant alterations in the null or inverse association between fish intake and NHL risk.
Tobacco smoking is a potential risk factor for NHL development.Citation3 In the current meta-analysis, whether or not adjusted by smoking status significantly altered the risk association between fish consumption and NHL risk (SRRs: 0.93 vs. 0.75; P for difference = 0.085), which indicates smoking status may be one of the confounding factors for this association.
Forth, a degree of publication bias is unavoidable since we included only articles published in English and small studies with null results tend to be unpublished. In fact, our analysis provided evidence for such bias according to Egger's test (P = 0.026). However, based on the further trim-and-fill method, no additional risk estimates were included to balance the funnel plot, and the summary risk estimates for this association remained unchanged.
In conclusion, from the present meta-analysis, we still cannot draw definite conclusion that fish consumption play a favorable role in the prevention of NHL, because our evidence is largely limited to case–control studies, whereas cohort studies have produced conflicting results. More epidemiological studies with prospective designs that control for important confounders and focus on the incidence of NHL relative to different levels of fish consumption, different types of fish in the diet are warranted to verify our findings.
Disclaimer statements
Contributors LY and DW designed the study; LY, WS, and DW conducted the search and data extraction; LY, WS, and XX collected the data; LY, XW, and LZ analyzed the data and wrote the manuscript. All authors read and approved the final content.
Funding None.
Conflicts of interest None.
Ethics approval Ethical approval is not required, because this manuscript is a meta-analysis.
supplementary_figure_2__1_.tif
Download TIFF Image (3.6 MB)supplementary_Figure_1__1_.tif
Download TIFF Image (1.7 MB)Reference
- American Cancer Society. Cancer facts and figures 2012. Atlanta, GA: American Cancer Society; 2012.
- Ekstrom-Smedby K. Epidemiology and etiology of non-Hodgkin lymphoma – a review. Acta Oncol. 2006;45:258–71. doi: 10.1080/02841860500531682
- Kamper-Jorgensen M, Rostgaard K, Glaser SL, Zahm SH, Cozen W, Smedby KE, et al. Cigarette smoking and risk of Hodgkin lymphoma and its subtypes: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph). Ann Oncol. 2013;24:2245–55. doi: 10.1093/annonc/mdt218
- Larsson SC, Wolk A. Body mass index and risk of non-Hodgkin's and Hodgkin's lymphoma: a meta-analysis of prospective studies. Eur J Cancer 2011;47:2422–30. doi: 10.1016/j.ejca.2011.06.029
- Rohrmann S, Linseisen J, Jakobsen MU, Overvad K, Raaschou-Nielsen O, Tjonneland A, et al. Consumption of meat and dairy and lymphoma risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2011;128:623–34. doi: 10.1002/ijc.25387
- Daniel CR, Sinha R, Park Y, Graubard BI, Hollenbeck AR, Morton LM, et al. Meat intake is not associated with risk of non-Hodgkin lymphoma in a large prospective cohort of U.S. men and women. J Nutr. 2012;142:1074–80. doi: 10.3945/jn.112.158113
- De Stefani E, Ronco AL, Deneo-Pellegrini H, Boffetta P, Correa P, Barrios E, et al. Meat, milk and risk of lymphoid malignancies: a case-control study in Uruguay. Nutr Cancer 2013;65:375–83. doi: 10.1080/01635581.2013.761255
- Qin B, Xun P, He K. Fish or long-chain (n-3) PUFA intake is not associated with pancreatic cancer risk in a meta-analysis and systematic review. J Nutr. 2012;142:1067–73. doi: 10.3945/jn.111.156711
- Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, et al. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol. 2007;166:1116–25. doi: 10.1093/aje/kwm197
- Han YJ, Li J, Huang W, Fang Y, Xiao LN, Liao ZE. Fish consumption and risk of esophageal cancer and its subtypes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2013;67:147–54. doi: 10.1038/ejcn.2012.213
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–63.
- Jing K, Wu T, Lim K. Omega-3 polyunsaturated fatty acids and cancer. Anticancer Agents Med Chem. 2013;13:1162–77. doi: 10.2174/18715206113139990319
- Mohammadi E, Rafraf M, Farzadi L, Asghari-Jafarabadi M, Sabour S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2012;21:511–8.
- Charbonneau B, O'Connor HM, Wang AH, Liebow M, Thompson CA, Fredericksen ZS, et al. Trans fatty acid intake is associated with increased risk and n3 fatty acid intake with reduced risk of non-hodgkin lymphoma. J Nutr. 2013;143:672–81. doi: 10.3945/jn.112.168658
- Chiu BC, Cerhan JR, Folsom AR, Sellers TA, Kushi LH, Wallace RB, et al. Diet and risk of non-Hodgkin lymphoma in older women. JAMA 1996;275:1315–21. doi: 10.1001/jama.1996.03530410029029
- Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90.
- Matsuo K, Hamajima N, Hirose K, Inoue M, Takezaki T, Kuroishi T, et al. Alcohol, smoking, and dietary status and susceptibility to malignant lymphoma in Japan: results of a hospital-based case-control study at Aichi Cancer Center. Jpn J Cancer Res. 2001;92:1011–7. doi: 10.1111/j.1349-7006.2001.tb01054.x
- Cross AJ, Ward MH, Schenk M, Kulldorff M, Cozen W, Davis S, et al. Meat and meat-mutagen intake and risk of non-Hodgkin lymphoma: results from a NCI-SEER case-control study. Carcinogenesis 2006;27:293–7. doi: 10.1093/carcin/bgi212
- Chiu BC, Dave BJ, Ward MH, Fought AJ, Hou L, Jain S, et al. Dietary factors and risk of t(14;18)-defined subgroups of non-Hodgkin lymphoma. Cancer Causes Control 2008;19:859–67. doi: 10.1007/s10552-008-9148-3
- Hu J, La Vecchia C, DesMeules M, Negri E, Mery L. Meat and fish consumption and cancer in Canada. Nutr Cancer 2008;60:313–24. doi: 10.1080/01635580701759724
- Ollberding NJ, Aschebrook-Kilfoy B, Caces DB, Wright ME, Weisenburger DD, Smith SM, et al. Phytanic acid and the risk of non-Hodgkin lymphoma. Carcinogenesis 2013;34:170–5. doi: 10.1093/carcin/bgs315
- Fritschi L, Johnson KC, Kliewer EV, Fry R. Animal-related occupations and the risk of leukemia, myeloma, and non-Hodgkin's lymphoma in Canada. Cancer Causes Control 2002;13:563–71. doi: 10.1023/A:1016331128897
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. doi: 10.1001/jama.283.15.2008
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. doi: 10.1136/bmj.327.7414.557
- Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9.
- Orsini N BR, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. doi: 10.2307/2533446
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. doi: 10.1136/bmj.315.7109.629
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
- Hu J, La Vecchia C, Morrison H, Negri E, Mery L. Salt, processed meat and the risk of cancer. Eur J Cancer Prev. 2011;20:132–9. doi: 10.1097/CEJ.0b013e3283429e32
- Chang ET, Smedby KE, Zhang SM, Hjalgrim H, Melbye M, Ost A, et al. Dietary factors and risk of non-hodgkin lymphoma in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14:512–20. doi: 10.1158/1055-9965.EPI-04-0451
- Chajes V, Torres-Mejia G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, et al. omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomarkers Prev. 2012;21:319–26. doi: 10.1158/1055-9965.EPI-11-0896
- Brinkman MT, Karagas MR, Zens MS, Schned AR, Reulen RC, Zeegers MP. Intake of alpha-linolenic acid and other fatty acids in relation to the risk of bladder cancer: results from the New Hampshire case-control study. Br J Nutr. 2011;106:1070–7. doi: 10.1017/S0007114511001346
- Prentice RL. Dietary assessment and the reliability of nutritional epidemiology reports. Lancet 2003;362:182–3. doi: 10.1016/S0140-6736(03)13950-5
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65.
- Guo X, Johnson RC, Deng H, Liao J, Guan L, Nelson GW, et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer. 2009;124:2942–7. doi: 10.1002/ijc.24293
- Goh KL, Cheah PL, Md N, Quek KF, Parasakthi N. Ethnicity and H. pylori as risk factors for gastric cancer in Malaysia: a prospective case control study. Am J Gastroenterol. 2007;102:40–5. doi: 10.1111/j.1572-0241.2006.00885.x
- Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am J Epidemiol. 2010;171:969–79. doi: 10.1093/aje/kwq032
- Davis DA, Archuleta MM, Born JL, Knize MG, Felton JS, Burchiel SW. Inhibition of humoral immunity and mitogen responsiveness of lymphoid cells following oral administration of the heterocyclic food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) to B6C3F1 mice. Fundam Appl Toxicol. 1994;23:81–6. doi: 10.1006/faat.1994.1082
- Sorensen IK, Mortensen A, Kristiansen E, van Kreijl C, Adamson RH, Thorgeirsson SS. Short-term carcinogenicity testing of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) in E(mu)-pim-1 transgenic mice. Carcinogenesis 1996;17:2221–7. doi: 10.1093/carcin/17.10.2221
- Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S; discussion 29S–31S.
- Tramacere I, Pelucchi C, Bonifazi M, Bagnardi V, Rota M, Bellocco R, et al. Alcohol drinking and non-Hodgkin lymphoma risk: a systematic review and a meta-analysis. Ann Oncol. 2012;23:2791–8. doi: 10.1093/annonc/mds013