Abstract
This Editorial provides a brief commentary exploring the possibilities of the bowel acting as a substitute for kidney function in chronic kidney disease/end stage renal disease. Three concepts are highlighted as potential means by which bowel function can serve as an alternative to renal filtration: oral sorbents, diarrhea therapy and bacterial-enzyme nitrogen recycling within the gut.
Willem Johan (‘Pim’) Kolff, who died a day before his 98th birthday on February 11, 2009, opened our current era of life extension via vital organ replacement when he invented the first practical artificial kidney in 1943. Acceptance of Kolff's vision of a bionic future, however, was highly limited. As recounted by Kolff, ‘When in 1929, I told the Chef de Clinic at the University of Groningen, the Netherlands, that I was going to work on an artificial kidney, his reaction was not only disbelief but he actually became angry’Citation1. Working in a semi-secret, isolated laboratory, under the German occupation of the Netherlands in World War 2, Kolff tested prototypes of his artificial kidney though 15 consecutive patient deaths, between 1943 and 1945, until his 16th ‘hemodialysis’ treated patient survived, changing our world. Kolff brought his device to the United States and, in a newly established Department of Artificial Organs at the Cleveland Clinic, turned his attention to fabricating a substitute artificial heart. In 1990, Life magazine designated Kolff as one of the 100 most important Americans of the 20th centuryCitation2.
Typical of the majority of artificial organ enthusiasts, convened at the 1964 founding meeting of the European Dialysis and Transplant Association (EDTA), Kolff enthusiastically predicted in a talk entitled ‘To Live Without Heart and Kidneys’, that: ‘The symbol of life, the site of love, and the habitat of the soul, the human heart, will be replaced by a mechanical pump’Citation3. After guiding medicine through the transformation of irreversible kidney failure from an absolute death sentence to a reason to seek treatment with one of several varieties of artificial kidneys, Kolff expressed the view that medical practice was ‘just around the corner from implantable self-sensing insulin pumps, artificial livers, and an implantable kidney’Citation4. A growing number of artificial organ visionaries predicted that the ‘doctor's office’ would soon be converted into a repair shop offering spare part replacement.
Absent from the early proceedings of newly established American and European societies devoted to reporting and discussing advances in the new field of ‘Artificial Organs’ was any consideration of the magnitude of the cost of treating all who might benefit from treatment with devices replacing vital organ function. American mass production – the basis for winning history's greatest war – followed by remarkable cost reduction for individual treatment with penicillin, and other wonder drugs, inhibited worry over any prohibitive expense of broad application of dialysis or artificial hearts. Industrialized nations, it was thought, should be able to extend renal replacement therapy, as its cost plummeted, to less wealthy nations making death in uremia a rarity.
Sadly, what actually happened throughout the world tells a different story. Just as the availability of a smallpox vaccine in 1796 did not lead to eradication of the disease until 19795, having the means to avert death in kidney failure has until this day been ineffectual for the large majority of those afflicted with the disease. Illustrating the point, the EDTA, in 1980, analyzed 14,084 incident patients with end-stage renal disease (ESRD) in 32 European countries, noting that ‘there was a strong linkage between the rate per million population treated for ESRD in 35 countries and the per capita gross national product in dollarsCitation6. Affluent nations treated ESRD care at a rate in excess of 200 per million (Japan, US, Switzerland), while poor nations were unable to treat more than 50 per million (South Africa, German Democratic Republic, Greece) (). Criticism of health care systems’ delivery of care for kidney failure included the United Kingdom, a democracy with socialized medicine ranking near the bottom, meaning that the majority of British uremic patients died without treatmentCitation7. Japan, by contrast, a nation devastated by war, permitted dialysis as a private practice business, topped the list. There was minimal hope that the world might treat kidney failure at the rate that enthusiasts promisedCitation8.
Figure 1. Selected national rates of incidence and prevalence of ESRD therapy as reported in the 2008 report of the United States Renal Data System10, the United States and Japan have the highest rates while Bangladesh illustrates the almost total absence of uremia therapy
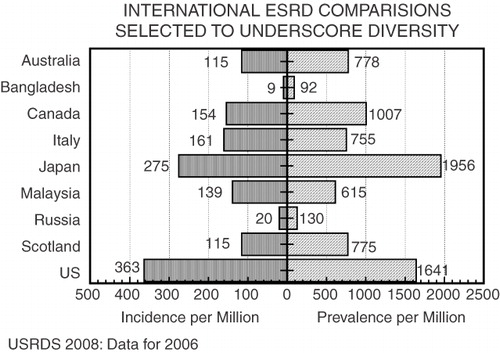
Fifteen years laterCitation9, continuing through todayCitation10, the stark reality that despite the existence of a means for its prevention, death without ESRD therapy is the unavoidable fate for most so afflicted. Death in ESRD like overall mortality is an inverse correlate of each nation's affluenceCitation11. In a cross-national examination of overall mortality in 25 developed countries, the key correlate of total death rate was each country's gross national product (GNP) and not the number of medical doctors, nurses, midwives, or hospital beds; nor was any association detected between total deaths and alcohol or tobacco consumption or military expenditureCitation12. That conditions might improve can be inferred from events after the collapse of Communism, in Eastern Europe, when dialysis treatment rates tripledCitation13 as a rise in GNP increased provision of health careCitation14. It follows that current regimens of ESRD treatment, for the foreseeable future, cannot be supported by the majority of underfunded national health care systemsCitation15, restricting application of present uremia regimens to affluent nationsCitation16.
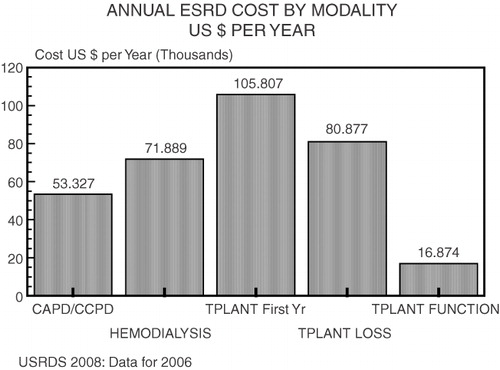
Given our current global recession, the objective for clinical nephrologists concerned with reducing death in kidney failure is to reduce the cost of treatment for ESRD (a year of hemodialysis requires $40,000 to $80,000 depending on country, ) by taking advantage of high technology. We have done this repeatedly in the past. As an example, the first quartz watches were priced above $3,000 when introduced; in 2009 they can be purchased for less than 50 cents. One direction of active investigation is the production of engineered cells programmed to replace failed pancreases, livers, kidneys and lungs.
Figure 2. The United States Renal Data System 2008 report10 illustrates how the annual cost of renal replacement therapy is linked to the therapeutic modality chosen, overall expenses in the first transplant year amounted to over $100,000 while each year of hemodialysis had Medicare reimbursement of $71,889; the range of overall application of ESRD therapy is sharply different in affluent versus developing countries as shown in Figure 3
Concepts of substitute kidneys and livers are regularly reported. Chang, over fifty years ago, suggested an additional option for organ replacementCitation17: design of semipermeable microcapsules as artificial cellsCitation18. Artificial cells use ultrathin polymer membrane envelopes, either as spherical membranes containing solutions or suspensions, or as a membrane coating on individual solid granules of adsorbentCitation19. Because of their large surface to volume relationship (e.g. 2.5 mCitation2 surface area in 10 ml of 20 m diameter or 300 ml of 2–5 mm diameter microcapsules) and their ultrathin membranes (for example, less than 0.05 micron) artificial cells transport permeant molecules at incredibly rapid rates. Multiple reports recount the value of oral microencapsulation to replace enzymes in genetic deficiencyCitation20, as well as living hepatocytesCitation21 to control hyperbilirubinemia in the Gunn ratCitation22.
Bowel as a kidney: oral sorbents
Another means of substituting for absent kidney function traces back through the beginnings of medicine indicating that employing the intestine may act as a substitute kidneyCitation23. As reviewed by ThompsonCitation24, Dioscorides’ Materia Medica in 40 BC advocated administration of terra sigillata, a sacred earth found on the Greek island of Lemnos, for multiple disorders including diseases of the kidney. Pliny in 100 AD prescribed this ‘esteemed medicine’ as an oral sorbent ‘against complaints of the spleen and kidneys, copious menstruation, also poisons and wounds caused by serpents’. Although terra sigillata is forgotten, other oral sorbents including charcoalCitation25, oxidized starchCitation26, locust bean gum (a mannose polymer derived from seeds of the ceratonia siliqua tree)Citation27, and microcrystalline carbon with an oxygen complex surface oxideCitation28 have each been reported in the 20th century as beneficial in the uremic syndrome by promoting nitrogenous waste extraction.
By 1960, several investigators documented the potential for nitrogen waste extraction from the human bowel. Schloerb, a surgeon at the Mayo Clinic, isolated a loop of ileum and by repeated perfusions with a lactated saline solution was able to prolong the life of otherwise fatally ill young individuals with chronic uremia for more than a yearCitation29. Not unexpectedly, Kolff had speculated on the value of extracting solutes via the gut as a substitute kidneyCitation30 both by lavage (dialysis) and by extraction using an oral sorbentCitation31. Sparks found that bowel fluid contained sufficient urea, creatinine, and uric acid to suggest that intestinal extraction might be clinically of valueCitation32 by means of ingestion of chemical ‘binders’ now termed sorbentsCitation33, testing a combination of activated charcoal and oxidized starch.
GiordanoCitation34 in Naples, Italy evaluated the periodic acid oxidation product of starch, dialdehyde starch (oxystarch), as a nitrogen sorbent in clinical trials combined with charcoalCitation35 reporting evidence that the bowel might indeed be a useful focus of efforts to remove nitrogenous wastes in uremia, a conclusion confirmed in Brooklyn, New YorkCitation36. Oxystarch under physiologic conditions binds urea at a capacity of 178–277 mmoles/mole of oxystarch aldehyde. Giordano's team maintained patients in advanced uremia using oxystarch at a dose of 30 to 40 g/day for over two years maintaining a constant blood urea nitrogen concentration while the serum creatinine gradually rose until the need for dialysis was inescapable.
More recently, AST-120, an oral sorbent comprised of particles of porous carbon with a diameter of 0.2 to 0.4 mm has attracted attention as a means of prolonging the interval until dialytic therapy is mandated. Preliminary studies in Sprague Dawley rats subjected to 4/5th nephrectomy and then treated with AST-120 1 g/day noted delay in onset of glomerular sclerosis while renal function is preservedCitation37. Miles et al. reported increased mean survival of 7/8ths nephrectomized, AST-120 treated rats to 104 days compared with 68 days in controlsCitation38. Clinical trials, thus far limited to Japan, of AST-120 in a dose of 3.2 to 7.2 g/day to 27 patients with renal insufficiency prolonged the interval between an azotemic patient's serum creatinine level reaching 6 mg/dl to the start of maintenance hemodialysis from a mean of 5.0 months in controls to a mean of 14.3 months while improving the severity of anemia. There have been no prospective, double-blind, alternate case evaluations of AST-120 in uremia. In Japan, in 2004, thousands of patients with progressive renal insufficiency are being treated with AST 120; Phase 2 studies are in progress in the US.
Diarrhea therapy
During the 1947 Egyptian cholera epidemic Captain Robert Allan Phillips (1906-1976) devised highly efficacious methods for intravenous fluid repletionCitation39. Subsequently, at the United States Naval Medical Research Unit (NAMRU)-2 in Taipei, Phillips designed a glucose-based oral cholera rehydration therapy to replace the then standard intravenous regimen. Recognizing that as a consequence of profound and sustained diarrhea, those afflicted with cholera evinced a sharp decrease in their plasma levels of nitrogen-containing wastes (urea, creatinine, uric acid), Phillips grasped the potential utility of induced diarrhea as a means of treating renal failure. Subsequently, Young et al. in Taipai, successfully introduced an oral kidney failure regimen consisting of hyperosmotic fluid containing mannitol 220 mMols/l administered at 240 ml every 5 min until a total of 7 l is reachedCitation40.
Diarrhea induced a urea clearance of 27.8 ml/min while bowel creatinine clearance reached 7.4 ml/min. Per three hour treatment session, a mean of 4931 mg of nonprotein nitrogen was removed, of which 3373 mg was urea nitrogen, during each diarrhea sessionCitation41. Bowel extraction of nitrogen during induced diarrhea is proportional to its initial level in plasma. Absent any dialysis program, Chinese uremic patients (creatinine clearance of 2–10 ml/min) were treated in Taipei with thrice weekly induced diarrhea lasting 3–7 hr for up to two years effecting symptomatic improvement with good tolerance of the regimen. All had improved appetite and reduced pruritus. When 17 uremic patients practiced diarrhea therapy at home on a thrice weekly 3 hr schedule for a mean of 6.8 months with a range of 1.3 to 16 months, a limit was reached when endogenous creatinine clearance fell to 1–2 ml/min when ‘nausea and vomiting gradually reappeared and signs of fluid retention set in’. No objective, controlled, randomized prospective studies of diarrhea therapy have been reported, though the concept is appealing, yet proof of efficacy is lacking. Especially noteworthy is the low cost of components of the diarrhea regimen which at the time of reporting was less than $3.00 per treatment, present costs would be about $9.00 per treatment.
Bacterial enzyme nitrogen recycling within the gut
Probiotic bacteria are commonly defined as live microorganisms, which, when administered in adequate amounts, confer a health benefit to the host. Elsewhere in this issue, a preliminary prospective double-blind trial of administration of programmed probiotic bacteria in patients with chronic kidney disease is reported. Underlying this approach to reducing the cost and extending availability of uremia therapy are several reports by veterinarians. Ruminant animals utilize cellulose and urea for nutrition because of bacterial metabolism within their intestinesCitation42 surviving weeks to months following bilateral nephrectomy in sheepCitation43, and cowsCitation44. Farmers depend on rumen-based chemical reactions when feeding urea and cellulose wastes to cattle who convert urea to essential amino acids using cellulose as an energy source. Dairy cows live for six generations, calve, and produce normal milk on a protein-free dietCitation45. Rumen microbial enzymes ‘learn’ to utilize urea and ammonium salts as sole sources of nitrogen permitting protein synthesis. After protein synthesis within the ruminant animal's gut, digestion and metabolism of protein following absorption are similar to that in non-ruminant animals.
Attempting to emulate ruminant physiology, Setälä, in Helsinki, administered enzymes extracted from cow feces to uremic patients basing the logic of his treatments on the hypothesis that bacterial enzymes utilize ammonia, potassium, phosphorus, and other nitrogenous wastes to cleave ‘vasoconstrictatory peptides in the intestines’Citation46. Setälä treated 10 uremic patients and 10 normal controls, with specifically extracted enzymes from cultured (immobilized or free) ‘pre-adapted’ ‘apathogenic’ soil microorganisms ‘trained’ to convert urea, creatinine, uric acid, guanidino derivatives, and other nonprotein nitrogen compounds (NPN) to amino acids utilizing ammonia, potassium, phosphorus, and other nitrogenous wastes. Azotemia and hypertension decreased significantly during treatment but then increased again after the regimen was discontinuedCitation47.
Based on the Setälä concept, Prakash and Chang continuously reduced blood urea levels in azotemic rats by instilling semipermeable microencapsulated genetically engineered live cells containing living urease-producing Escherichia coli DH548. Previously, these workers demonstrated that genetically engineered E. coli DH5 contain the urease gene from K. aerogens that metabolizes urea without production of ammonia. Pursuing this approach, bacteria engineered to catabolize unexcreted nitrogenous wastes within the intestine – termed probiotic bacteria – after testing in azotemic rats and miniature pigs, are now being fed to patients with CKD in a multicenter clinical trialCitation49.
Conducted in an outpatient nephrology clinic in Scarborough, Ontario, Canada, 13 outpatients with CKD Stage 3 or 4 first 3-month treatment period, were randomly assigned to group A or B and provided capsules containing either placebo or a probiotic formulation (90 billion CFU/day, 15 billion/gel cap, 2 caps × 3/day). In the second phase of the study, Groups A and B switched treatment for 3 months. Variables monitored included blood urea nitrogen (BUN), serum creatinine, phosphorus, and uric acid as well as responses to a self-administered quality of life questionnaire. Symptomatic complaints attributed to CKD decreased during administration of probiotic bacteria. From this preliminary trial, it appears feasible to expand study of probiotic formulations as adjunctive health supplements to help stabilize and maintain quality of life in CKD stage 3 and 4 patients. Expanded clinical trials of gut-based probiotic bacteria to determine their value as a component of renoprotection in progressive CKD may assist in sustaining life quality.
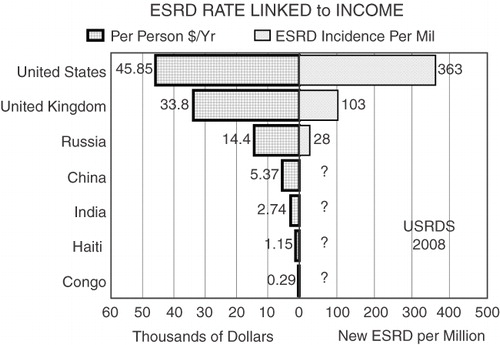
An intragut bacterial enzyme-based treatment for uremia in 2009 perspective is neither novel nor an overly optimistic expression of science fiction. Absent Chang's or other fresh directions in uremia research, our inability to improve the lot of most people with failing kidneys will persist far into this century resulting in tens of thousands of deaths forced by socioeconomic realities (). Redefining artificial organs to encompass hybrid devices, smart cells, and even fractional products of engineered cells and bacteria is a practical necessity and a pragmatic reality.
Figure 3. Linkage between annual per person income and incident ESRD treatment rate is depicted for several nations compared with the United States10, broadly applying any therapy requiring thousands of dollars per year is beyond the budget of both nations with a population of over one billion (China, India) and limited industrialization (Congo, Haiti)
Transparency
Declaration of funding
This Editorial was prepared independently, without any funding, as a result of an invitation from the CMRO editorial office.
Declaration of financial/other relationships
E.A.F. has disclosed that he serves, without compensation, as Chair of Kibow Biotech's Scientific Advisory Board, and that the Renal Division of his institution is currently in receipt of research funding for a clinical trial sponsored by Kibow Probiotics.
All peer reviewers receive honoraria from CMRO for their review work. Peer Reviewer 1 has disclosed that he/she is a scientific consultant on clinical trials for Jamieson Laboratories Inc. Peer Reviewer 2 has disclosed that he/she has no relevant financial relationships.
Acknowledgments
None.
References
- Kolff WJ. A visiting scientist looks at the Arab refugee problem. Salt Lake City, Utah: Division of Artificial Organs, University of Utah, 1979: p.1
- Life Magazine, Fall 1990, The most important Americans of the 20th Century
- Kolff WJ. To live without heart and kidneys. Proc Eur Dial Transplant Assoc 1968;1:21–24
- Friedman EA. Feasibility of a bionic kidney. Proc Int Conf Cybernetics Soc 1976;189–194
- Smallpox: 30th Anniversary of Global Eradication, United States Public Health Service, Center for Disease Control. Available at: www.cdc.gov/Features/SmallpoxEradication. [Last accessed June 5, 2009]
- Jacobs C, Broyer M, Brunner FP, et al. Combined report on regular dialysis and transplantation in Europe, XI 1980. Proc European Dialysis Transplant Assoc 1980;18:4-58
- Berlyne GM. Over 50 and uremic equals death. The failure of the British National Health Service to provide adequate dialysis facilities. Nephron. 1982;31(3):189–90
- Friedman EA, Delano BG. Can the world afford trauma therapy? In Proceedings of the 8th International Congress on Nephrology. Athens Greece: S. Karger, 1981: pp. 677–83
- Friedman EA. Facing the reality: the world cannot afford uremia therapy at the start of the 21st century. Artif Organs 1998;19:481-5
- U.S. Renal Data System, USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Chapter 12, International Comparisons. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008
- Friedman EA. Nephrology and the rationing of health care. Contrib Nephrol 1993;102:230-36
- Poikolainen K, Eskola J. Health services resources and their relation to mortality from causes amenable to health care intervention: a cross-national study. Int J Epidemiol 1988;17:86-9
- Friedman EA. Revelations behind a fallen curtain: dialysis restriction and the Berlin wall. Nephrol Dial Transplant 1994;9:242-3
- Valek A, Wing AJ. Development of dialysis and transplant activity in the world during the seventies of the 20th century. Z Exp Chir Transplant Künstliche Organe 1984;17:86-9
- Friedman EA. ESRD therapy: an American success story. JAMA 1996;275:1118-22
- Friedman EA, Delano BG. Can the world afford uremia therapy? Proc 8th Internat Cong Nephrol. Athens: S. Karger, 1981: pp. 677-83
- Chang TM. 50th anniversary of artificial cells: their role in biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation, cell/stem cell therapy and nanorobotics. Artif Cells Blood Substit Immobil Biotechnol 2007;35(6):545-54
- Chang TMS. Semipermeable microcapsules. Science 1964;146:524-7
- Chang TM, MacIntosh FC, Mason SG. Semipermeable aqueous microcapsules. 1. Preparation and properties. Can J Physiol Pharmacol 1996;44:115-28
- Safos S, Chang TM. Enzyme replacement therapy in ENU2 phenylketonuric mice using oral microencapsulated phenylalanine ammonia-lyase: a preliminary report. Artif Cells Blood Substit Immobil Biotechnol 1995;23:681-92
- Liu ZC, Chang TM. Preliminary study on intrasplenic implantation of artificial cell bioencapsulated stem cells to increase the survival of 90% hepatectomized rats. Artif Cells Blood Substit Immobil Biotechnol 2009;8:1-5
- Bruni S, Chang TM. Effect of donor strains and age of the recipient in the use of microencapsulated hepatocytes to control hyperbilirubinemia in the Gunn rat. Int J Artif Organs 1995;18:332-9
- Friedman EA. Bowel as a kidney substitute in renal failure. Amer J Kidney Dis 1996;28:943-50
- Thompson CJS. Terra Sigillata, a famous medicament of ancient times. Trans XVII Internat Med Congress 1914; London, Sect 23;433-40
- Yatzidis H. Recherches sur l’épuration extra rénale à l'aide du charbon actif. Nephron 1964;1:310-12
- Giordano C, Esposito R, Randazzo G, et al. Oxystarch as a gastrointestinal sorbent in uremia, in Kluthe R, Berlyne G, Burton B (eds) Uremia. Stuttgart: Georg Thieme Verlag, 1972: pp. 231-9
- Yatzidis H, Koutsicos D, Digenis P. Newer oral sorbents in uremia. Clin Nephrol 1979;2:105-6
- Niwa T, Yazawa T, Ise M, et al. Inhibitory effect of oral sorbent on accumulation of albumin-bound inoxyl sulfate in serum of experimental uremic rats. Nephron 1991;57:84-8
- Schloerb PR. Intestinal dialysis for kidney failure. Personal experience. ASAIO Trans 1990;36(1):4-7
- Kolff WJ. New ways of treating uremia. London: J. A. Churchill, 1947
- van Noordwijk J. Early History in Kampen Dialysis & Transplantation 1982;11:15-18
- Sparks RE, Mason NS, Meier PM, et al. Binders to remove uremic waste metabolites from the GI tract. Trans Am Soc Artif Intern Organs 1972;18:458-64
- Sparks RE, Mason NS, Meier PM, et al. Removal of uremic waste metabolites from the intestinal tract by encapsulated carbon and oxidized starch. Trans Am Soc Artif Intern Organs 1971;17:229-38
- Giordano C, Esposito R, Demma G. The possibility of reducing the blood nitrogen level in humans by the administration of a polyaldehyde. Bull Soc Ital Biol Sper 1968;44:2232-4
- Giordano C, Esposito R, Pluvio M. Oxycellulose and ammonia-treated oxystarch as insoluble polyaldehydes in uremia. Kidney Int Suppl 1975;Feb(3):380-2
- Friedman EA, Fastook J, Beyer MM, et al. Potassium and nitrogen binding in the human gut by ingested oxidized starch. Trans Amer Soc Artif Internal Organs 1974;20:33-43
- Okada K, Takahashi S. Correction by oral adsorbent of abnormal digestive tract milieu in rats with chronic renal failure. Nephrol Dial Transplant 1995;10:671-6
- Miles AM, Fleishacker J, Hyppolite G, et al. Oral sorbent (AST-120) slows uremia progression in 7/8th nephrectomized rats (Abstract). J Amer Soc Nephrology 1995;6:1024
- SavarinoSJ. A legacy in 20th-century medicine: Robert Allan Phillips and the taming of cholera. Clin Infect Dis 2002;35(6):713-20
- Young TK, Lee SC, Tang CK. Diarrhea therapy of uremia. Clin Nephrol 1979;11(2):86-91
- Young TK, Lee SC, Tai LN. Mannitol absorption and excretion in uremic patients regularly treated with gastrointestinal perfusion. Nephron 1980;25:112-6
- Korhonen M, Ahvenjarvi S, Vanhatalo A, et al. Supplementing barley or rapeseed meal to dairy cows fed grass-red clover silage: II. Amino acid profile of microbial fractions. J Anim Sci 2002;80:2188-96
- Singh J, Singh AP, Peshin PK, et al. Studies on the effects of total nephrectomy in sheep. Can J Comp Med 1983;47:217-21
- Biochemical changes following bilateral nephrectomy in the bovine. Watts C, Campbell JR. Res Vet Sci 1970;11:508-14
- Virtanen AI. Milk production of cows on protein-free feed. Science 1966;53:1603-14
- Setälä K. The promise of enzymes in therapy of uremia. I. Theoretical basis – bowel physiology. Nephron 1984;37:1-6
- Setälä K. Bacterial enzymes in uremia management. Kidney Int Suppl 1978;8:S194-202
- Prakash S, Chang TMS. Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nature Med 1996;2:883-7
- Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin 2009;25:1919-30
