Abstract
Background:
Although chronic pain affects around 20% of adults in Europe and the USA, there is substantial evidence that it is inadequately treated. In June 2009, an international group of pain specialists met in Brussels to identify the reasons for this and to achieve consensus on strategies for improving pain management.
Scope:
Literature on chronic pain management was reviewed, and information presented to and discussed by a panel of experts.
Findings:
It was agreed that guidelines are not universally accepted by those involved in pain management, and pain treatment seems to be driven mainly by tradition and personal experience. Other factors include poor communication between patients and physicians, the side effects of analgesic drugs, and limited individualisation of therapy. Difficulty in maintaining the balance between adequate pain relief and acceptable tolerability, particularly with strong opioids, can lead to the establishment of a ‘vicious circle’ that alternates between lack of efficacy and unpleasant side effects, prompting discontinuation of treatment. The medical community’s understanding of the physiological differences between nociceptive pain and neuropathic pain, which is often more severe and difficult to treat, could be improved. Increasing physicians’ knowledge of the pharmacological options available to manage these different pain mechanisms offers the promise of better treatment decisions and more widespread adoption of a multi-mechanistic approach; this could involve loosely combining two substances from different drug classes, or administering an analgesic with two different mechanisms of action. In some circumstances, a single compound capable of addressing both nociceptive and neuropathic pain is desirable.
Conclusions:
To improve patient outcomes, a thorough understanding of pain mechanisms, sensitisation and multi-mechanistic management is required. Universal, user-friendly educational tools are therefore required to familiarise physicians with these topics, and also to improve communication between physicians and their pain patients, so that realistic expectations of treatment can be established.
Introduction
Pain is the most common reason why patients seek medical attention, and presents a serious problem for a large proportion of the population. In the USA, chronic pain affects 25% to 30% of all AmericansCitation1 and nearly 60% of adults aged over 65 years with pain report that it has lasted 1 year or moreCitation2. Among 46 394 adults in 15 European countries and Israel, the prevalence of chronic pain was recently found to be 19%Citation3, which is consistent with the results of surveys in AustraliaCitation4, DenmarkCitation5 and NorwayCitation6.
Guidelines have been developed for the treatment of chronic pain based on the World Health Organization’s three-step ‘Cancer pain relief with a guide to opioid availability’Citation7. Based on the intensity of pain, these recommend weak opioids, such as codeine and tramadol, for Step II, and strong opioids for Step III. Newer guidelines focus on treating pain according to the mechanism or mechanisms involved; i.e. neuropathic, nociceptive or a combination of bothCitation8. Despite the existence of these international and national guidelines, however, their incorporation into daily practice is marginalCitation9,Citation10, and substantial evidence indicates that pain is inadequately treatedCitation11. A recent systematic review which pooled data from 52 studies found that the prevalence of pain was >50% in all cancer typesCitation12. The authors concluded that despite the clear WHO recommendations cancer pain is still a major problemCitation12. In the pan-European survey mentioned above, 21% of the respondents with chronic pain had suffered for more than 20 years, 40% were not satisfied with the management of their pain, and 12% said their physician never determined how much pain they were experiencingCitation3. An observational study in Germany found that the median time between the beginning of pain and an appointment in a specialised pain centre was 12 yearsCitation13. A 2008 IMS analysis of analgesic consumption data also shows a huge variation in the use of analgesics across Europe; for example, opioids are more frequently prescribed in northern countries, and less frequently in the south and east. This similarly suggests that many patients with pain do not receive adequate therapy, although hard evidence about the level of suffering is lacking and further research is needed.
There are various reasons for the under-treatment of chronic pain, including a lack of awareness of the extent of the problem among healthcare providers, allied to an incomplete understanding of the pathophysiology of chronic pain. One major factor relates to the prescription of opioids, which is still influenced by medical, ethical, cultural and legislative considerationsCitation14,Citation15. Physicians’ and patients’ misconceptions about these analgesics mean that strong opioids are often not prescribed despite being indicated, or the doses prescribed are insufficient. The agents themselves also have relative disadvantages that limit their success in long term treatment; these include the potential development of tolerance and hyperalgesia, lack of reliable efficacy in difficult to treat pain syndromes, and unpleasant gastrointestinal and CNS side effects. These have the effect of reducing patient compliance, which itself contributes significantly to the problem of under-treatment. The risk of addiction also needs to be addressed before prescribing opioids, especially when these drugs are used to treat non-malignant pain.
Charles Darwin’s quotation, “It is not the strongest species that survive, nor the most intelligent, but the ones most responsive to change” can also be applied to many different forms of human endeavour. If the medical community is to address the unmet needs of patients suffering from severe chronic pain, it must change various aspects of current practice. In order to clarify issues responsible for the inadequacy of current treatment, and to suggest possible ways forward, an international panel of pain experts met at the CHANGE PAIN Advisory Board meeting in Brussels on June 7th and 8th 2009. Present were consultant physicians specialising in pain management and international opinion leaders with long experience in the field. There was no audience. A further objective was to achieve consensus on the specific strategies to be adopted.
Methods
In May 2009, prior to the meeting, the literature on chronic pain management was screened through a database search of PubMed, using the key words: chronic pain management guidelines neuropathic pain adults (seven articles); chronic pain management guidelines cancer pain adults (41 articles); and pain pathways review chronic pain adults (35). Altogether, 83 articles were surveyed; 42 were reviewed, and information extracted from them was presented to the expert panel. Panel members were also asked to contribute additional articles and information for review. Various types of literature were used, and it was not stratified according to the level of evidence; this is a possible area of improvement for a future review. Throughout the meeting, various aspects of the treatment of chronic pain management were discussed and summarised, resulting in a consensus of opinion. The resultant discussion-based report was then submitted to each expert panel member for approval.
Note that an updated search of the literature relating to the keywords published since the meeting (carried out in the period December 2009 – January 2010) found an additional eight research articles; these were not considered by the panel.
Issues responsible for inadequate treatment
Guidelines versus clinical practice
There are two broad categories of pain: nociceptive and neuropathic. Nociceptive pain is adaptive and biologically useful, contributing to survival by protecting the organism from injury or promoting healing when injury has already occurred. By contrast, neuropathic pain is maladaptive, has no association with a noxious stimulus, and has its origin in an abnormality of the somatosensory nervous systemCitation16.
Chronic pain, which has lost its direct relationship to the triggering event and has become a disease in its own right, presents a special therapeutic challenge. It is a multifactorial condition which manifests both physical and psychological symptoms, including anxiety and depression, reduced mobility, and sleep and appetite disturbancesCitation3,Citation17–19. These symptoms produce a measurable reduction in patients’ quality of lifeCitation20 and necessitate frequent use of healthcare resources, generating a substantial socioeconomic burdenCitation21. In most medical disciplines, pain is more than merely a symptom; it should be considered as a disease entity in its own right, involving biological, psychological and social aspects, which can influence the outcome of medical and surgical treatmentCitation22. Clinicians should still, however, seek to identify and treat the basic pain mechanism.
Several surveys have shown that chronic pain affects approximately 20% of the population in developed countriesCitation1,Citation3–6. Although cancer pain accounts for only 0.1% to 1% of moderate to severe pain, the prevalence of pain in cancer patients is high and varies according to the stage of the disease; it is experienced by 50% to 70% of patients receiving active therapy and 60% to 84% of patients with late-stage diseaseCitation23–27. Back pain accounts for the highest proportion (approximately 50%) of moderate to severe chronic painCitation3. In the case of chronic low back pain, only around one third of sufferers experience pure nociceptive pain, with another third having neuropathic pain and the final third having mixed pain, with both nociceptive and neuropathic symptomsCitation28. Recent studies have shown that when a neuropathic component is present pain is perceived to be more severeCitation29.
There are numerous international guidelines on the treatment of chronic pain from bodies such as the WHOCitation7, the European Society for Medical Oncology (ESMO)Citation30 and the European Federation of Neurological Societies (EFNS)Citation31. National societies and working groups also produce valuable guidance. The advice provided is similar in many respects, although there are differences in the drug regimens recommended.
The WHO guidelines were originally developed for the treatment of cancer pain, but are now used extensively for non-cancer pain as wellCitation7. The three-step WHO pain ladder is based on the intensity of the patient’s pain, and suggests the following regimen:
Step I: A non-opioid analgesic should be used for moderate pain, with co-analgesics if necessary.
Step II: If pain persists or increases, a weak opioid may be added.
Step III: If pain still persists, then a change should be made to a strong opioid.
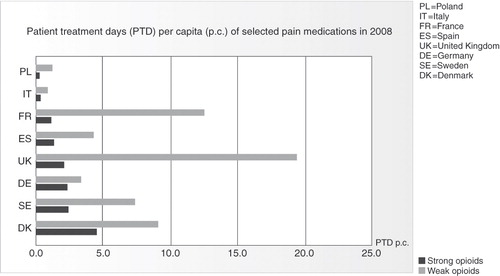
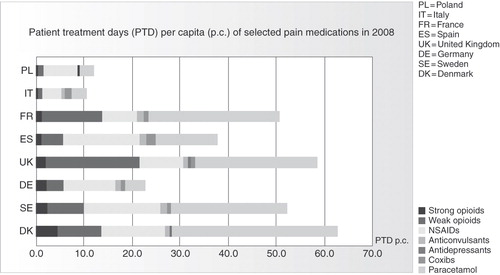
Despite the consistency of authoritative guidelines on pain management, data for the year 2008 from IMS Health (International Medical Statistics), an internationally accepted information provider, shows huge differences in the consumption of analgesics across Europe, both in quantity and the type of agent prescribed (). Similar differences exist in the use of opioids, with physicians in northern Europe much more willing to prescribe them than physicians in the south and east (). In the Breivik study, for example, the consumption of strong opioids was close to 0% in Italy and 12% to 13% in the United Kingdom and IrelandCitation3. This indicates, firstly, that the guidelines are not being widely followed, and may even be unavailable to some clinicians, and pain management is probably less than optimal for some patientsCitation32. Secondly, the main factors determining analgesic usage appear to be tradition and personal experienceCitation33, although local health authority rules may influence the treatment provided. This may reflect the low level of influence of clinical recommendations and guidelines, particularly among clinicians who are not pain specialists.
Figure 1. Differences in analgesic consumption across Europe. Source: IMS, year 2008. Figure shows PTD per capita for selected European countries. Basis for calculation: sum of sold units of selected analgesics converted into PTDs based on average daily consumption. For anticonvulsants and antidepressants, only their use for pain treatment is considered.
Consensus point
The multifactorial nature of chronic pain may not be fully appreciated – treatment seems to be driven mainly by tradition and personal experience.
Limitations of current pain therapy
The typical chronic pain patient has a lengthy medical history, has consulted numerous physicians, experienced several ineffective therapies and is likely to suffer from concomitant depression and sleeping disorders. The overall effect of chronic pain on quality of life is greater than that of many other chronic disorders. The most prevalent single condition is back painCitation3, which has been shown to constitute a common, debilitating and costly health problemCitation34,Citation35, and is the leading cause of disability in people below the age of 45 in the USACitation9,Citation36. The socioeconomic burden of back pain is indicated by its annual cost to the German economy – €48 billion. Of this sum, only 28.3% is directly incurred by medical treatment; 71.7% of the overall cost is attributable to sick leave and disabilityCitation37.
Participants of the consensus meeting agreed that one reason for the high prevalence of patients with chronic pain – and its current treatment often being ineffective – is lack of educationCitation38. Unless the mechanisms and treatment of chronic pain become mandatory topics in medical schools and specialist training, the number of patients suffering from chronic pain will remain high. Many physicians are taught about acute pain but may not be familiar with the processes of peripheral and central sensitisation, in which the response to a stimulus increases as a result of repeated or continuous exposure. Furthermore, there may be a lack of awareness of the role of genetic, psychological and social factors in the development of chronic pain.
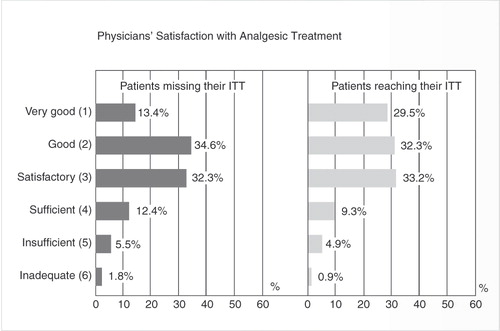
Another important factor in the ineffective treatment of chronic pain is inadequate communication between physician and patientCitation38. This makes it difficult for the clinician to understand the patient’s situation fully. When comparing assessments of the degree of pain-related impairment, fewer than 20% of physicians’ ratings have been shown to match those of their patientsCitation39. As the patients in this study were being seen every day, the result implies that over 80% of physicians had an inaccurate picture of their patients’ symptoms, and how they were changing over timeCitation39. This communication gap contributes to clinicians and patients often having very different expectations of treatment. In one study, physicians assessed the treatment success of two groups of patients; one of patients who considered they had failed to reach their individual treatment targets, and the other of patients who considered they had achieved their individual treatment targets. On a six-point scale, the physicians considered the success rates in the two groups to be almost identical, despite the difference in the patients’ own assessments ()Citation39.
Modern pain management analyses the mechanisms which contribute to a patient’s pain and determines analgesic therapy accordingly, enhancing endogenous pain-control systems if possible. Furthermore, the multi-modal treatment of chronic pain incorporates not only this approach to pharmacological treatment, but also non-pharmacological strategies such as interventional pain management, physiotherapy, psychotherapy and pain rehabilitation.
Where non-steroidal anti-inflammatory drugs (NSAIDs) are indicated in chronic pain because there is an inflammatory component, exposure to these agents should be minimised, as recommended by the European Medicines Agency in their 2005 guidelinesCitation40. In fact, recently updated American Geriatrics Society (AGS) Management Guidelines for older patients with persistent pain recommend considering the use of opioids for all patients with moderate to severe pain, pain-related functional impairment, or diminished quality of life due to painCitation41. Furthermore, the AGS has stated that NSAIDs and COX-2 inhibitors should be considered rarely, and with extreme caution, in highly selected individualsCitation41. These principles call into question the continuing validity of the WHO pain ladder, which recommends treatment solely on the basis of pain intensity and recommends NSAIDs for Step I. For truly multi-modal management of chronic pain, guidelines are needed that incorporate a range of equally important therapies, in order to take into account the different mechanisms responsible.
Measurement of pain
The experience of pain is individual and so is the degree of pain relief obtained from a given therapy. Communication between physician and patient can be improved by using standardised instruments, such as pain scales and questionnaires, to gain an insight into the individual’s pain and the level of pain relief that would be acceptable. This varies considerably; some patients may prefer to accept a higher level of pain in return for fewer side effects, while others consider analgesia to be of primary importance. Attempting to set arbitrary targets, such as ‘a 50% reduction in mean pain score’ or ‘a 30% reduction in pain intensity’, as is necessary in clinical trials to establish efficacy, is not always meaningful in clinical practice and should not be the primary objective. Individual patients have individual treatment targets and expectations, which should be established as part of pain management. Identifying and meeting them is the criterion for effective treatmentCitation42.
Consensus point
Owing to inadequate communication between physicians and patients, pain treatment is less likely to be effective unless individual targets are set.
The Vicious Circle in the treatment of severe chronic pain
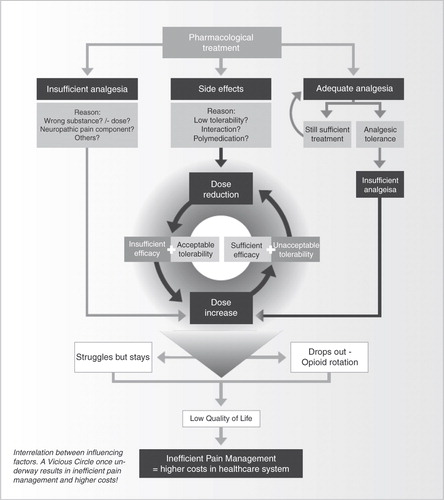
Currently, the pharmacological management of severe chronic pain is often inefficient because of the difficulty in maintaining the balance between adequate pain relief and acceptable tolerability, as well as tailoring the treatment to the mechanism of the pain. This may be explained by the hypothesis of the Vicious Circle, which is particularly applicable to classical opioids but also plays a role in combination therapy ().
The Vicious Circle in clinical practice
If tolerability is satisfactory but the level of analgesia is insufficient, the dose is usually increased in order to achieve satisfactory pain relief. As the efficacy of classical opioids is dose-dependent, the increase may produce effective analgesia – but it also increases the risk of side effects. Tolerability may then become unacceptable, or symptomatic medication (e.g. anti-emetics to combat nausea and vomiting) may prove ineffective, leading the physician (or patient) to reduce the dose of opioid. This improves tolerability but compromises analgesic efficacy, and a dose increase is considered. Thus treatment begins to go round in circles and the Vicious Circle continues. One solution could be to switch to an alternative opioid, but this requires the clinician to recognise the Vicious Circle before its negative consequences become established. Other medications can be prescribed specifically to target the troubling side effect, but this may lead to drug–drug interactions associated with polypharmacy, or may introduce additional side effects related to the new medication. Moreover, patient compliance may be less than with single drug therapy and treatment costs will increaseCitation43.
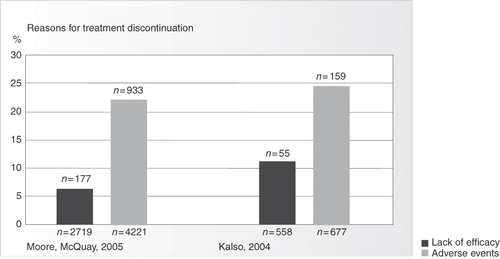
The Vicious Circle, and its continuous imbalance between analgesia and tolerability, is a burden to patients and results in poor treatment outcomes. The quality of life of patients is frequently impaired by inadequate pain relief or unacceptable side-effects, or both. As a result, many patients discontinue their opioid analgesic treatment, principally because of gastrointestinal and CNS side effectsCitation43,Citation44. Systematic reviews have shown that 20%–30% of patients who received opioids for chronic non-cancer pain in controlled clinical trials discontinued their treatment, principally as a result of adverse eventsCitation45,Citation46 (). One analysis of 34 registered clinical trials found that 20% of subjects withdrew from treatment because of adverse events, whereas only 6.5% withdrew because of lack of efficacyCitation46. However, most of these patients received opioids for moderate rather than severe pain and the trials were of relatively short duration. This suggests that discontinuation rates are probably higher in clinical practice and the application of these results to the uncontrolled outpatient setting is consequently limited.
Lack of efficacy and the Vicious Circle
Insufficient analgesia is primarily due to the specific agent prescribed (lack of potency or too low a dose), but may be evoked by other factors, including side-effects, drug–drug interactions, and the development of tolerance. Where pain has a neuropathic component, the analgesic potency of opioids may be limited by pathophysiological mechanisms; opioid receptors are downregulated and dynorphin and cholecystokinin are released, reducing opioid responsiveness and producing relative opioid toleranceCitation47. However, several randomised, controlled clinical trials have demonstrated that neuropathic pain does respond to opioidsCitation48, although higher doses are required than for nociceptive pain, and these may initiate the Vicious Circle. Co-analgesics such as anticonvulsants and antidepressants are often required if the condition is chronic, because effective pharmacological treatment of this type of pain often requires more than one mechanism of action. Ad hoc or loose-dose combinations of analgesic agents intuitively make sense but have an inherent problem associated with their use: i.e. difficulty maintaining the dose-ratio within the optimal rangeCitation49. Potent agents with a broad efficacy covering both nociceptive and neuropathic components in a single molecule would therefore represent an outstanding improvement in the current management of severe chronic pain.
The most important enzyme system for metabolising drugs is the cytochrome P-450 enzyme system, which can vary between patients because of genetic polymorphism and is responsible for almost 50% of the overall elimination of common drugs, including beta blockers, selective serotonin reuptake inhibitors (SSRIs) and cholesterol-lowering agentsCitation50,Citation51. Opioids which are mainly metabolised via this pathway are therefore susceptible to reduced reliability of analgesia – these include frequently prescribed agents such as tramadol and codeineCitation52. They are susceptible to the action of other drugs which may either inhibit or induce the enzyme system and thus affect the overall analgesic performance of the opioidCitation52. Therefore opioids which avoid this pathway are favoured in the treatment of polypharmacy patients. Other factors which increase the likelihood of the Vicious Circle becoming established are the development of analgesic tolerance, which involves the dose–response curve shifting to the right and affects about 15% of patients receiving parenteral opioidsCitation53, and opioid-induced hyperalgesia.
Side effects and the Vicious Circle
The management of opioid-related side effects remains a major clinical challenge. Reducing the dose of systemic opioid risks starting the Vicious Circle of pain management, while alternative approaches – switching opioids, managing adverse symptoms or changing the route of administration – not only have a negative impact on the patient but demand additional resources and medication. For example, patients may require anti-emetics or a co-analgesic with a different mechanism of action, or more frequent consultations with healthcare professionals. It should also be noted that some side effects of opioids, such as nausea and vomiting, are transient, while others, such as constipation, may become permanent.
One systematic review of 15 randomised clinical trials in non-cancer patients receiving opioids for moderate to severe pain found that approximately 80% experienced one or more adverse events, the most common being constipation (41%) and nausea (32%)Citation45. It has been suggested that constipation is the most bothersome side effect, both in terms of its impact on daily life and the frequency of occurrenceCitation54,Citation55, but nausea and vomiting have been described as highly distressing by patients, sufficient to make them reduce their opioid dose or stop taking it completelyCitation56,Citation57. Sedation affects between 20% and 70% of patients receiving opioidsCitation58, but patients may also experience other CNS side effects including cognitive and psychomotor impairment, hallucinations, and toxic effects on neurons, such as hyperalgesia and analgesic toleranceCitation53. Less frequently reported are pruritus, respiratory depression, immunological suppression and hormonal changes. All these opioid-induced side effects are a burden to both the individual and society, but their wider impact is probably underestimated by healthcare professionalsCitation59, despite the fact that they reduce compliance and lead to treatment discontinuations.
Prescribers and patients usually react to inadequate pain relief or the development of analgesic tolerance by increasing the dose, thereby increasing the risk of dose-related side effects and establishment of the Vicious Circle. The co-administration of other drugs can produce pharmacokinetic and pharmacodynamic drug–drug interactions, which may also reduce tolerability. For example, two co-administered substances may evoke similar adverse events and have a cumulative effect, or there may be an increase in the serum concentration of active metabolites. Evidence suggests that the metabolites of several opioids (e.g. tramadol, codeine, tilidine) account for a considerable proportion of their clinical effectsCitation60. Conversely, co-administration may produce a reduction of active substances in the plasma, compromising analgesic efficacy. One factor influencing a compound’s potential for these drug–drug interactions is the degree to which it binds to serum transport proteinsCitation61 and it is particularly important in pain patients, who are often receiving multiple medications.
Thus polypharmacy is linked to adverse drug reactions, particularly with respect to drug metabolismCitation62. One study reported that 67% of patients on opioids are taking ≥1 other non-opioid prescription drugCitation63, with >59% of people over 65 taking ≥5 different drugs/week and >15% taking ≥10 different drugs/weekCitation64. Individual dose titration and the avoidance of adverse effects, owing to either the accumulation or lack of formation of active metabolites, are important considerations when opioids are usedCitation65. Opioids are subject to different metabolic processes and clinicians need to be aware that many opioids have active metabolites that may become therapeutically important; for example, in cases of renal failure or genetic polymorphisms of drug-metabolizing enzymes.
It can be concluded that current treatment options for chronic pain are restricted by a reduced efficacy in pain with a neuropathic component, poor tolerability, and limited treatment reliability as a result of drug–drug interaction, polymedication, dose instability and the development of analgesic tolerance. All these contribute to the inefficiency of current pain management and the unmet needs of patients. In addition, there is evidence that the pharmacological management of chronic non-cancer pain in the general clinical situation is much less effective than is suggested by randomised, controlled trialsCitation66.
Consensus points
Pharmacological treatment is often limited by the side effects of the drugs used; treatment with strong opioids is often limited by the Vicious Circle, because side effects often limit the effective analgesic dose that can be achieved.
High doses of analgesics or combination therapy may lead to side effect problems.
Contributing factors that drive the Vicious Circle include side effects, lack of efficacy or analgesic tolerance, which may all lead to treatment discontinuation.
Increasing awareness of the Vicious Circle among the medical community could reduce treatment discontinuation.
Current treatment options
The core strategy of pharmacological chronic pain treatment is to reduce sensitisation, decrease pain amplification and restore normal pain thresholds, which requires a multi-mechanistic approach. Some conditions, such as low back pain and cancer pain, frequently have both nociceptive and neuropathic components which respond differently to different types of currently used analgesicCitation67.
An analysis of pain medication showed that chronic pain patients in Europe are most frequently prescribed NSAIDs (76% of patients), but in 70% of these cases the therapy had to be changed because of inadequate pain controlCitation19. There is also evidence that NSAIDs are preferably chosen in the place of opioids for long-term treatment of non-cancer pain, especially in some southern European countriesCitation3. The mainly peripheral action of NSAIDs is to inhibit prostaglandin synthesis via the inhibition of cyclooxygenase (COX), where they act mainly on inflammatory nociceptive pain and not the neuropathic component. As the protective function of prostaglandins is diminished or abolished, there is an increased risk of gastrointestinal erosions and ulcers, and also of renal failureCitation68,Citation69. The high level of plasma protein binding means the risk of drug–drug interactions is correspondingly highCitation70. Thus the use of NSAIDs in severe chronic pain may lead to serious side effects and inadequate pain relief. Furthermore, recent recommendations of the European Medicines Agency (EMEA) suggest limiting the use of NSAIDs to the shortest possible duration at the lowest possible doseCitation40.
Opioids comprise the current standard treatment for severe chronic pain and are classified according to their analgesic potency. Weak opioids, such as codeine and tramadol, are used for moderate pain, while severe pain is treated with strong opioids such as morphine and fentanyl. They act by binding to opioid receptors in the CNS and peripheral organs, the main receptor types being µ, κ and δ. For example, morphine inhibits the release of several different neurotransmitters, including acetylcholine, glutamate and substance P, by its action on the µ-opioid receptorCitation71.
At the synapses, opioids reduce pain signal transmission at two sites. Activation of pre-synaptic opioid receptors reduces intracellular cyclic adenosine monophosphate (cAMP) concentration and calcium ion influx, inhibiting the release of the excitatory neurotransmitters, glutamate and substance P. At the post-synaptic neuron, opioid binding produces a hyperpolarisation of the neuronal membrane, thereby reducing the probability of an action potential being generated. Opioids also affect supraspinal structures of pain processing, especially the thalamus and limbic systemCitation72. The emotional assessment of pain is altered via these higher centres, so that it is still perceived but no longer experienced as unpleasant or threatening. The side effects of opioids, and their implication in the Vicious Circle, have already been discussed.
Anticonvulsants were originally developed to treat cerebral seizures, and their analgesic effect may be produced by the same mechanismsCitation73. The main indication for these drugs in pain therapy is trigeminal neuralgia. Although anticonvulsants include agents of various pharmacological classes, with different mechanisms of action, they all inhibit neuronal excitation and stabilise nerve membranes. Gabapentin has been shown to be effective against neuropathic painCitation31,Citation74,Citation75. It acts by binding to a subunit of pre-synaptic voltage-dependent calcium channels, which reduces the release of pre-synaptic transmittersCitation76. In combination with morphine it increases pain tolerance in healthy volunteersCitation77 and has also been shown to reduce patients’ need for opioids in postoperative pain with a neuropathic componentCitation78. Pregabalin, which is also recommended for neuropathic pain conditionsCitation31,Citation74,Citation75, produces analgesia by interacting with special N-type calcium channels in the brain and spinal cordCitation79. This reduces the excessive release of neurotransmitters such as glutamate and substance P. However, side effects such as sedation, dizziness, ataxia, peripheral oedema, nausea and liver toxicity can be problematic; one study found that anticonvulsants produced significant side effects justifying the discontinuation of treatment in 26% of child and adolescent patientsCitation80.
Tricyclic antidepressants (TCAs) inhibit the neuronal reuptake of noradrenaline or serotonin from the synaptic cleftCitation81. The resultant increase in neurotransmitter concentration intensifies activity in the descending pain inhibition pathway, producing analgesia. TCAs also affect histaminergic, cholinergic and glutaminergic neurotransmission, and block sodium channelsCitation81. These agents are recommended for the first-line treatment of neuropathic pain although they are not registered for pain reliefCitation31,Citation74,Citation75, and are also used in cases of complex regional pain syndrome (CRPS) and tension headache. The onset of analgesia usually precedes the antidepressant effect (3–7 days vs 2–3 weeks)Citation82. Also, pain relief requires a lower dose than treatment for depression. The side effects of tricyclic antidepressants may be classified as followsCitation31,Citation83: anticholinergic (dry mouth, disturbed vision, constipation), cardiovascular (orthostatic hypotension, palpitation, tachycardia, disturbed conduction), CNS (dizziness, sedation, insomnia, tremor, convulsion, myoclonus) and others (impaired liver function, anaphylaxis).
Serotonin and noradrenaline reuptake inhibitors (SNRIs) selectively inhibit the reuptake of serotonin and noradrenaline from the synaptic cleft, producing analgesia in a similar way to tricyclic antidepressantsCitation84. Two SNRIs, duloxetine and venlafaxine, have been successfully tested in peripheral polyneuropathy, but are considered a second-line treatment because of moderate efficacy. Unlike TCAs, SNRIs have no affinity for adrenergic, cholinergic or histaminergic receptors and do not induce the corresponding side effectsCitation85. Thus tolerability is better and they are safer to use in patients with cardiac diseaseCitation31. Side effects of duloxetine include nausea, vomiting, constipation and somnolence, while those of venlafaxine include agitation, diarrhoea and increased liver enzymesCitation31.
When stimulated, peripheral nociceptors transmit pain signals to the dorsal horn of the spinal cord, and then to the thalamus and the cerebral cortex via ascending pathways. In a normal pain response, the intensity of pain increases with the intensity of the stimulusCitation86,Citation87. Analgesics exert their effects at different points on the pain pathways, indicating that the use of two or more agents with different mechanisms of action increases the probability of interrupting pain signals and relieving painCitation86,Citation87. Some combinations (e.g. opioids with local anaesthetics or COX inhibitors) can demonstrate additive or synergistic effectsCitation88,Citation89. One potentially promising approach is to combine opioid receptor agonism with noradrenaline reuptake inhibitionCitation90. General efficacy might be increased by a loose combination of the appropriate agents, but this risks increasing the incidence and complexity of side effects. Alternatively, the two mechanisms can be combined in a single molecule to produce a synergistic effectCitation90.
Consensus point
NSAIDs and COX-2 inhibitors should be given at the lowest effective dose for the shortest possible duration to control symptoms, as outlined in the EMA and AGS guidelines.
Treatment of pain with both a neuropathic and nociceptive component
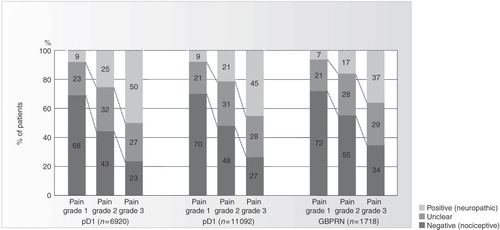
Typical conditions which manifest both nociceptive and neuropathic pain components include cancer pain and chronic back pain. It is estimated that in approximately two thirds of back pain patients there is a neuropathic componentCitation28, and this has a considerable effect on the nature and course of the conditionCitation91; it is more common in patients with severe chronic low back pain and associated with both higher pain intensity and a longer duration of sufferingCitation28. Three epidemiological studies have shown that the presence of neuropathic components correlates with pain severity, and that in the majority of cases of severe back pain (pain grade 3: ≥5 on NRS and functional ability ≤70) a neuropathic component is involvedCitation29 (). There is also an economic impact; among patients with persistent back pain, the costs incurred by a patient with neuropathic pain are higher than those of an average patient, and as much as 67% higher than those of a patient with purely nociceptive painCitation29. Knowledge of the accurate diagnosis of neuropathic pain symptoms and their effective treatment is therefore crucial.
In addition to the ascending pathways described earlier, which transmit pain signals from peripheral nociceptors to the thalamus and cerebral cortex, there are also descending pathways. These enable the cortex and subcortical areas of the brain to modulate the experience of pain by sending impulses via the periaqueductal grey and CNS tracts to the dorsal horn of the spinal cord segment where painful stimuli are being transmitted. Pain facilitatory and inhibitory cells (‘on’ and ‘off’ cells) in the rostral ventromedial medulla (RVM) modify the transmission of these impulses, increasing or decreasing the level of painCitation92. A variety of treatment options exist which influence these pathways in different ways. Combination therapy is commonly used to treat chronic back pain with nociceptive and neuropathic pain components, using opioids with anticonvulsants, but this approach increases the risk of side effects and drug–drug interactionsCitation93.
A primary market research study was conducted between March and May 2009 by GFK Healthcare, Nürnberg, among 996 physicians concerned with the treatment of severe chronic and acute painCitation33. These comprised general practitioners, pain specialists, rheumatologists/orthopaedists and oncologists/palliative care specialists in seven European countries (Germany, UK, France, Spain, Italy, Sweden, Denmark). Although the physicians’ general strategy approximated to the WHO pain ladder (NSAIDs or paracetamol, followed by weak opioids, anticonvulsants or TCAs, and then strong opioids) the results indicate that the treatment of severe chronic back pain differs markedly from one country to another. For example, in Germany these patients are treated with a combination of an opioid and one or two co-medications for neuropathic pain, and the most frequently prescribed opioids are tilidine, tramadol and transdermal fentanyl. Multiple medications are used in the UK, including TCAs, anticonvulsants and strong opioids, although 39% of patients receive codeine. The Spanish approach is to give strong opioids, possibly with an anticonvulsant, while very few opioids, especially strong opioids, are prescribed in Italy. Tramadol is incorporated into treatment at an early stage in France. Several interviewees in Denmark would not use weak opioids but commence opioid treatment with morphine, contrasting with the strong usage of weak opioids in Sweden. This wide range of approaches reflects the difficulty of relieving pain which has a neuropathic component.
Consensus points
In severe chronic low back pain, there tends to be a neuropathic component which may require the use of strong opioids, and combination therapy is often applied.
Pain which has a neuropathic component is often more severe and more difficult to treat.
Minimising opioid-induced side effects
Several novel pharmacological strategies are currently being pursued to increase the tolerability of opioids and thereby increase patient compliance, with the objective of reducing the unmet needs of chronic pain patients. These include the concomitant use of opioids and µ-opioid receptor antagonists, as well as the combination of two analgesic mechanisms of action in a single molecule.
One recent approach to reducing side effects, specifically constipation, is to combine the oral administration of a classical opioid and a µ-opioid receptor antagonist. The underlying rationale is that the antagonist competes with the opioid for the opioid receptors in the gutCitation94. A prolonged-release (PR) oxycodone/naloxone combination has been launched in Germany and has now gained European approval. Limited published data indicate that the combination improves the bowel function index (BFI) when compared with oxycodone aloneCitation95. One double-blind, multicentre trial in an out-patient setting enrolled 322 adult patients with moderate-to-severe, non-cancer pain and demonstrated a significant improvement in BFI scores with oxycodone PR/naloxone PR compared with oxycodone PR after 4 weeks of treatment (−26.9 vs. −9.4, respectively; p < 0.0001), without compromising analgesiaCitation95. However, limited information is available regarding the impact on discontinuation rates, and further studies are required to provide evidence of its long term efficacy.
Newly developed peripheral µ-opioid receptor antagonists, which have no analgesic function, have been used for the symptomatic treatment of opioid-induced bowel dysfunction (OIBD) and constipationCitation96. Alvimopan has gained FDA approval for short term use in hospitalised patients with postoperative ileus, but two clinical trials have found its efficacy in OIBD to be inconsistentCitation96,Citation97. Methylnaltrexone has FDA approval for treating opioid-induced constipation, and EU approval for the same indication in palliative care patients. In a 13-day clinical trial, intravenous methylnaltrexone produced a significantly higher percentage of laxation responses than placebo on each day of treatment, but this was accompanied by an increase in side effectsCitation98.
Other interesting compounds currently being developed include tapentadolCitation90. This is a novel, centrally acting analgesic that combines two mechanisms of action – µ-opioid agonism and noradrenaline reuptake inhibition – in a single molecule, which has recently been approved in the USA for the treatment of moderate to severe acute pain in patients 18 years of age and older; market authorisation applications for chronic moderate to severe and severe pain have been submitted in the US and Europe, respectively. Activation of the µ-opioid receptor inhibits presynaptic afferent input, while the increased level of noradrenaline in the synaptic cleft activates α-2 receptors and thereby inhibits the activity of second order neuronsCitation90. The rationale is that noradrenaline reuptake inhibition provides a proportion of the analgesic effect, so that a lower dose of opioid can be used to achieve a given level of analgesia; i.e. an ‘opioid-sparing’ effect. Clinical studies in patients with low back pain or arthritic pain have shown that tapentadol produces significantly less nausea, vomiting and constipation than equianalgesic doses of oxycodoneCitation99,Citation100. In addition, discontinuations owing to gastrointestinal and CNS side effects were 71% and 51% lower, respectively, in the patients who received tapentadol. The reduced incidence of GI side effects is thought to result from an opioid-sparing effect described earlier in this paper; a separation from oxycodone at tapentadol doses higher than 50 mg has been shown in most clinical trials performed.
Discussion
Knowledge of pain pathways and their relevance to the various treatment options is limited among the medical community. Although most pain patients are seen by general practitioners, who are in a position to prevent the development of the social and psychological problems that are characteristic of chronic pain, they often lack the ability to diagnose the presence of a neuropathic pain component. One consequence is that primary care physicians may not be able to identify severe pain that is likely to become chronic, in order to instigate appropriate early treatment. All too often the acute pain paradigm is addressed and patients are prescribed a series of ineffective analgesics. In the case of severe chronic low back pain, awareness that this condition frequently has a neuropathic component is also limited. Investigation of the pain mechanism should be complemented with treatment of the neuropathic pain component.
Developing educational tools to raise physicians’ awareness and knowledge of the ascending and descending pain systems, sensitisation, and the use of multi-modal pain management strategies would improve outcomes for patients. In particular, sufferers from neuropathic pain or pain with a neuropathic component would receive earlier and more effective treatment. The tools must be user-friendly and should focus on mechanisms rather than guidelines, as well as the individualisation of treatment. Thus there is also a need to improve clinicians’ communication with patients, awareness of investigation techniques, and provision of access to expert consultation.
Consensus points
There is limited awareness of the physiological difference between neuropathic and nociceptive pain, and the specific pharmacological options, within the medical community.
Improving this knowledge could lead to better treatment decisions; educational tools must be universal and user-friendly.
Conclusions
Chronic pain is a multifactorial condition, with both physical and psychological symptoms, that affects around 20% of the population in the developed worldCitation3. Pain management strategies vary widely between different countries. It is essential to improve education about chronic pain among healthcare professionals, and communication between physicians and patients, as well as increasing the individualisation of treatment.
Also, the pharmacological management of chronic pain is often compromised by the difficulty of maintaining a balance between adequate pain relief and acceptable tolerability, which can be explained by the Vicious Circle. Gastrointestinal and CNS side effects of classical opioids lead to high rates of withdrawalCitation43. The utility of these agents is also restricted by reduced efficacy in neuropathic pain, and limited reliability resulting from drug–drug interaction, dose instability and the development of analgesic tolerance. The pharmacological management of chronic pain often requires a multi-mechanistic approach, complemented by non-pharmacological therapies, particularly when there is a neuropathic component; this is associated with increased severity and longer duration of pain, as a result of sensitisation and elevation of normal pain thresholdsCitation47. Currently, true multi-modal pain management is often not instituted, and the presence of a neuropathic component often not identified.
Thus change is clearly desirable. The unmet needs of patients with severe chronic pain are evident; the prevalence and duration of suffering are too high and too few patients are satisfactorily treated. Greater agreement about the best treatment strategy for this condition is needed – with the focus on the individual patient – and it should be applied more consistently. Specifically, the following aspects of current practice must change:
the education of physicians and patients regarding pain management
the assessment of pain
awareness of the relevance of a neuropathic component in severe chronic pain
the pharmacological approaches directed at severe chronic pain.
Consensus points
The multifactorial nature of chronic pain may not be fully appreciated – treatment seems to be driven mainly by tradition and personal experienceCitation33.
Owing to the lack of communication between physicians and patients, pain treatment is less likely to be effective unless individual targets are set.
Pharmacological treatment is often limited by the side effects of the drugs used; treatment with strong opioids is often limited by the Vicious Circle, because side effects often limit the effective analgesic dose that can be achieved.
High doses of analgesics or combination therapy may lead to side effects.
Contributing factors that drive the Vicious Circle include side effects, lack of efficacy or analgesic tolerance, which may all lead to treatment discontinuation.
Increasing awareness of the Vicious Circle among the medical community could reduce treatment discontinuation.
NSAIDs and COX-2 inhibitors should be given at the lowest effective dose for the shortest possible duration to control symptoms, as outlined in the EMA and AGS guidelines.
In severe chronic low back pain, there tends to be a neuropathic component which may require the use of strong opioids, and combination therapy is often applied. The pain mechanism should also be investigated.
Pain which has a neuropathic component is often more severe and more difficult to treat.
There is limited awareness of the physiological difference between neuropathic and nociceptive pain, and the specific pharmacological options, within the medical community.
Improving this knowledge could lead to better treatment decisions; educational tools must be universal and user-friendly.
Transparency
Declaration of funding
This article was supported by an unrestricted educational grant from Grünenthal GmbH, Aachen, Germany.
Declaration of financial/other relationships
All authors have disclosed that they received honoraria from Grünenthal for attending the International CHANGE PAIN Advisory Board meeting in Brussels.
The CMRO peer reviewers 1 and 2 have received honoraria for their review work on this manuscript. Both have disclosed that they have no relevant financial relationships.
Acknowledgements
The authors thank Derrick Garwood Ltd, Cambridge, UK, for editorial support, which was sponsored by Grünenthal GmbH, Aachen, Germany.
This Commentary is based on the Proceedings of the International CHANGE PAIN Advisory Board Meeting, held in Brussels, Belgium, on June 7th and 8th, 2009.
References
- Coda BA, Bonica JJ. General considerations of acute pain. In: Loeser JD, Butler SH, Chapman CR, Turk DC, eds. Bonica’s Management of Pain, 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:222-40
- New Report Finds Pain Affects Millions of Americans. Atlanta, GA: Centers for Disease Control and Prevention, 2006. Available at: http://www.cdc.gov/nchs/pressroom/06facts/hus06.htm [Last accessed 9 July 2009]
- Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333
- Blyth FM, March LM, Brnabic AJM, et al. Chronic pain in Australia: a prevalence study. Pain 2001;89:127-34
- Sjøgren P, Ekholm O, Peuckmann V, et al. Epidemiology of chronic pain in Denmark: an update. Eur J Pain 2009;13:287-92
- Rustoen T, Wahl AK, Hanestad BR, et al. Prevalence and characteristics of chronic pain in the general Norwegian population. Eur J Pain 2004;8:555-65
- World Health Organization. Cancer Pain Relief with a Guide to Opioid Availability, 2nd edn. Geneva: World Health Organization, 1996
- Dworkin R, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237-51
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet 1999;354:581-5
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008;8:287-313
- Pain in the elderly with cancer. Cancer Pain Release, 2000, Volume 13, No. 2. Geneva, Switzerland: WHO Pain & Palliative Care Communications Program. Available at: https://whocancerpain.bcg.wisc.edu/index?q=node/34 [Last accessed 20 February 2010]
- van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49
- Schulte E, Hermann K, Berghöfer A, et al. Referral practices in patients suffering from non-malignant chronic pain. Eur J Pain 2009: published online 30 June 2009, doi:10.1016/j.ejpain.2009.05.015
- Bhamb B, Brown D, Hariharan J, et al. Survey of select practice behaviours by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin 2006;22:1859-65
- Jacobsen R, Sjøgren P, Møldrup C, et al. Physician-related barriers to cancer pain management with opioid analgesics: a systematic review. J Opioid Manag 2007;3:207-14
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32:1-32
- Becker N, Bondegaard Thomsen A, Olsen AK, et al. Pain epidemiology and health related quality of life in chronic non-malignant pain patients referred to a Danish multidisciplinary pain center. Pain 1997;73:393-400
- Gore M, Brandenburg NA, Hoffman DL, et al. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain 2006;7:892-900
- Rodriguez MJ. Survey of therapeutic attitudes towards patients with chronic pain in Spanish Pain Units. Rev Soc Esp Dolor 2006;13:525-32
- McDermott AM, Toelle TR, Rowbotham DJ, et al. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 2006;10:127-35
- Dunn KM, Croft PR. Epidemiology and natural history of low back pain. Eura Medicophys 2004;40:9-13
- Siddall PJ, Cousins MJ. Persistent pain as a disease entity: implications for clinical management. Anesth Analg 2004;99:510-20
- Brescia FJ, Portenoy RK, Ryan M, et al. Pain, opioid use, and survival in hospitalized patients with advanced cancer. J Clin Oncol 1992;10:149-55
- Vuorinen E. Pain as an early symptom in cancer. Clin J Pain 1993;9:272-8
- Portenoy RK, Kornblith AB, Wong G, et al. Pain in ovarian cancer patients. Prevalence, characteristics, and associated symptoms. Cancer 1994;74:907-15
- Donnelly S, Walsh D. The symptoms of advanced cancer. Semin Oncol;1995:22:67-72
- Caraceni A, Portenoy RK. An international survey of cancer pain characteristics. International Association for the Study of Pain. Pain 1999;82:263-74
- Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20
- Schmidt CO, Schweikert B, Wenig CM, et al. Modelling the prevalence and cost of back pain with neuropathic components in the general population. Eur J Pain 2009;13:1030-5
- Jost L, Roila F. Management of cancer pain. ESMO clinical recommendations. Ann Oncol 2008;19:ii119-21
- Attal N, Cruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 2006;13:1153-69
- Victor TW, Alvarez NA, Gould E. Opioid prescribing practices in chronic pain management: guidelines do not sufficiently influence clinical practice. J Pain 2009;10:1051-7
- CHANGE PAIN – Treatment Differences. Results of a Market Research Study. Grünenthal GmbH, Aachen. Available at http://www.change-pain.com/grt-change-pain-portal/GRT-CHANGE-PAIN-PORTAL_Home/Why_Change/Treatment_Differences/86600048.jsp [Last accessed 19 January 2010]
- Collins JJ, Baase CM, Sharda CE, et al. The assessment of chronic health conditions on work performance, absence, and total economic impact for employers. J Occup Environ Med 2005;47:547-57
- McNeil JM, Binette J. Prevalence of disabilities and associated health conditions among adults – United States, 1999. CDC Morb Mortal Wkly Rep 2001;50:120-5
- Workers’ Compensation Report. Kew, Victoria: Journal of Risk and Insurance, 1 November 2004
- Wening CM, Schmidt CO, Kohlmann T, et al. Costs of back pain in Germany. Eur J Pain 2009;13:280-6
- Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. J Am Board Fam Pract 2001;14:211-18
- Mueller-Schwefe GHH, Ueberall MA. Pain intensity of patients. Abstracts of the 11th World Congress on Pain. Sydney, 2005
- European Medicines Agency. Opinion of the committee for medicinal products for human use pursuant to article 5(3) of regulation (EC) no 726/2004, for nonselective non steroidal anti-inflammatory drugs (NSAIDs). Available at: http://www.emea.europa.eu/pdfs/human/opiniongen/nsaids.pdf [Last accessed 4 October 2009]
- Kuehn BM. New pain guideline for older patients: avoid NSAIDs, consider opioids. JAMA 2009;302:19
- Ueberall MA, Mueller-Schwefe G. Individual treatment targets in chronic pain management. Eur J Pain 2006;10:S184
- Harris J-D. Management of expected and unexpected opioid-related side effects. Clin J Pain 2008;24:S8-13
- Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med 2009;10:654-62
- Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372-80
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005;7:R1046-51
- Davis MP. What is new in neuropathic pain?. Support Care Cancer 2007;15:363-72
- Ballantyne JC, Shin N. Efficacy of opioids for chronic pain. A review of the evidence. Clin J Pain 2008;24:469-78
- Pergolizzi J, Wills LM. Multimodal analgesic therapy. In: Shorten G, Carr DB, Harmon D, et al., eds. Postoperative Pain, an Evidence-based Guide to Practitioners. Philadelphia, PA: Saunders Elsevier, 2006:182-96
- Drug Interactions Website. Available at: http://medicine.iupui.edu/flockhart/table.htm [Last accessed 20 June 2009]
- Wilkinson GR. Drug metabolism and variability among patients in drug response. NEJM 2005;352:2211-21
- Holmquist GL. Opioid metabolism and effects of cytochrome P450. Pain Med 2009;10:S20-9
- Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous systems of palliative care patients. J Pain Palliat Care Pharmacother 2007;21:15-25
- McNichol E. Opioid side effects. International Association for the Study of Pain. Pain: Clinical Updates 2007;15:1-6
- Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med 2009;10:35-42
- Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. J Clin Pract 2007;61:1181-7
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008;11:S105-20
- Villars P, Dodd M, West C, et al. Differences in the prevalence and severity of side effects based on type of analgesic prescription in patients with chronic cancer pain. J Pain Symptom Manage 2007;33:67-77
- McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004;11:3-9
- Lötsch J. Opioid metabolites. J Pain Symptom Manage 2005;29:10-24
- Kneip C, Terlinden R, Beier H, et al. Investigations into the drug–drug interaction potential of tapentadol in human liver microsomes and fresh human hepatocytes. Drug Metabolism Letters 2008;2:67-75
- Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother 2006;4:36-41
- National Disease Therapeutic Index (NDTI). Available at: www.ndti.org [Last accessed 25 October 2007]
- Patterns of Medication use in the United States. Boston, MA: Slone Epidemiology Center, 2005
- Coller JK, Christrup L, Somogyi A. Role of active metabolites in the use of opioids. Eur J Clin Pharmacol 2009;65:121-39
- Moulin DE, Morley-Forster PK, Connolly B, et al. Prospective study of the pharmacologic management of chronic neuropathic non-cancer pain. Eur J Pain 2007;11:S82
- Hawthorn J, Redmond K. Pain: Causes and Management. Oxford: Blackwell Sciences, 1998
- Chutkan R, Toubia N. Effect of nonsteroidal anti-inflammatory drugs on the gastrointestinal tract: diagnosis by wireless capsule endoscopy. Gastrointest Endosc Clin N Am 2004;14:67-85
- Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999;106:13S-24S
- Ito K, Iwatsubo T, Kanamitsu S, et al. Prediction of pharmacokinetic alterations caused by drug–drug interactions: metabolic interaction in the liver. Pharmacol Rev 1998;50:387-412
- North RA, Williams JT. How do opiates inhibit neurotransmitter release?. Trends Neurosci 1983;6:337-9
- Dickenson AH. Mechanisms of the analgesic actions of opiates and opioids. Br Med Bull 1991;47:690-702
- Fabian R. Der chronische Schmerz. Schriftenreihe der Bayerischen Landesapothekerkammer, 1999
- Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 2003;60:1524-34
- Finnerup NB. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118:289-305
- Bayer K, Ahmadi S, Zeilhofer HU. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology 2004;46:743-9
- Eckhardt K, Ammon S, Hofmann U, et al. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg 2000;91:185-91
- Dirks J, Fredensborg BB, Christensen D, et al. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology 2002;97:560-4
- Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel α2-δ (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007;73:137-50
- Harbord MG. Significant anticonvulsant side-effects in children and adolescents. J Clin Neurosci 2000;7:213-16
- Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399-409
- Tzschentke T. NA and 5-HT reuptake inhibitors and α2 agonists. In: Buschmann H, ed. Analgesics: From Chemistry and Pharmacology to Clinical Application. Weinheim, Germany: Wiley-VCH 2002:265-84
- Mutschler E, Geisslinger G, Kroemer HK, et al. Arzneimittelwirkungen. Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH, 2008
- Lambert O, Bourin M. SNRIs: mechanism of action and clinical features. Expert Rev Neurother 2002;2:849-58
- Scott ALE. New classes of antidepressant drugs. Adv Psych Treat 1999;5:104-11
- Kehlet H, Dahl JB. The value of ‘multimodal’ or ‘balanced analgesia’ in postoperative pain treatment. Anesth Analg 1993;77:1048-56
- Gottschalk A, Schroeder F, Ufer M, et al. Amantadine, a N-methyl-D-aspartate receptor antagonist, does not enhance postoperative analgesia in women undergoing abdominal hysterectomy. Anesth Analg 2001;93:192-6
- Vaughan CW. Enhancement of opioid inhibition of GABAergic synaptic transmission by cyclo-oxygenase inhibitors in rat periaqueductal grey neurones. Br J Pharmacol 1998;123:1479-81
- Christie MJ, Connor M, Vaughan CW, et al. Cellular actions of opioids and other analgesics: implications for synergism in pain relief. Clin Exp Pharmacol Physiol 2000;27:520-3
- Tzschentke TM, Christoph T, Kögel B. (–)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 2007;323:265-76
- Morlion B. The relevance of neuropathic components in severe chronic pain. CHANGE PAIN News and Reviews, 2009. ISSN 2041-8558
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol 2001;85:280-6
- Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain 2008;12:804-13
- Meissner W, Leyendecker P, Müller-Lissner S, et al. A randomised controlled trial with prolonged-release oral oxycodone and naloxone to prevent and reverse opioid-induced constipation. Eur J Pain 2008;13:56-64
- Simpson K, Leyendecker P, Hopp M, et al. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin 2008;24:3503-12
- Webster L, Jansen JP, Peppin J, et al. Alvimopan, a peripherally acting mu opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain. Pain 2008;137:428-40
- Paulson DM, Kennedy DT, Donovick R, et al. Alvimopan: an oral, peripherally acting, µ-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction – a 21-day treatment-randomized clinical trial. J Pain 2005;6:184-92
- Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008;358:2332-43
- Afilalo M, Oh C, Okamoto A, et al. Tapentadol immediate release compared with oxycodone immediate release for the relief of moderate-to-severe pain in patients with end stage joint disease. J Pain 2008;9:32
- Hale M, Upmalis D, Okamoto A, et al. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr Med Res Opin 2009;5:1095-104