Abstract
This commentary critically evaluates the limitations of genetic risk predictions in multifactorial disease, with specific reference to age-related macular degeneration (AMD). AMD is a common blinding disease with 33 million people worldwide experiencing vision impairment. Although gene polymorphism combinations infer an up to 50-fold increased risk of developing the disease, we are far from predicting AMD based on genetics. In the case of complex multifactorial disease such as AMD, to have the same predictive certainty that exists for monogenic disorders, we must account for all gene–environment interactions. We discuss sensitive vision tests that reflect causal gene–environment mechanisms and their potential in AMD risk prediction.
Introduction
Advancements in genomics research generate expectations about opportunities for predicting disease. In age-related macular degeneration (AMD)Citation1, a common blinding disease with 33 million people worldwide experiencing vision impairmentCitation2, gene polymorphism combinations imply an up to 50-fold increased risk of developing the diseaseCitation3–5. AMD is, however, a complex multifactorial disease including many non-genetic and environmental risk factors. As such, risk gene combinations together with AMD specific environmental risk and non-genetic factors are now being used to establish risk predictions for developing AMDCitation5–7. We outline the problem of multifactorial disease risk prediction in clinical practice and its limitations in AMD. We infer from evidence that sensitive, quantitative clinical vision measures that reflect causal relationship between gene and environment may increase the power of existing prediction models. This is critical because AMD risk is thought to be modifiable and vision measures could be used to better predict AMD than genetics alone and to directly assess lifestyle interventions and their effect on disease progression.
Prediction models of AMD risk
The genetics of AMD are unlike the genetics of monogenic diseases such as cystic fibrosis which is almost totally predictive, although the severity of cystic fibrosis may be modified by environmental factors including exposure to lung infections or other background genetics like inflammatory response genesCitation8. In the case of complex multifactorial diseases, to have the same predictive certainty that exists for monogenic disorders, we must account for all or the vast majority of the genes contributing to AMD risk and their interaction with all environmental risk factors. Unfortunately, we are far from this knowledge, which is supported by findings that about 40% of people who have developed AMD have a low genetic risk profile whereas only 20% of AMD sufferers have a very high genetic riskCitation5. Guidelines and genetic risk measures are urgently needed to help interpret genetic results, particularly as we enter an area where direct gene testing is becoming available to the consumerCitation9.
Statistical models using multivariate regression models that include genetic risk variants, clinical features of AMD and environmental risk factors have been very useful in estimating a risk for developing AMD and its progression of intermediate to advanced formsCitation6–7,Citation10. For example a large US multicenter study demonstrated that increased body mass index (BMI), smoking and high-risk genotypes for both CFH Y402H and ARMS2 A69S conferred a 19-fold higher risk of AMD progression compared to low genetic risk non-smokers with a lower BMICitation7. Another study showed that those with two high-risk CFH Y402H variants who are obese or smoke have a 12-fold and 9-fold increased risk, respectively, compared with persons who do not have the genetic variant or the modifiable risk factors, while smokers with two high-risk ARMS2 A69S variants have a 22-fold higher risk compared with non-smokers, making smoking a factor that almost doubles the riskCitation5. Risk of developing AMD can be predicted by combining genetic risk with smoking status and for example not smoking can reduce the prevalence of severe AMD by 33%Citation10. CFH increases the severity of geographic atrophy and ARMS2 the severity of choroidal neovascularizationCitation6.
No study has longitudinally evaluated a healthy cohort with no clinical signs of AMD but with genetic and environmental risk to directly test the predictors. The outcomes of risk prediction studies are limited due to the fact that risk models are directly applicable only to the (hyperselected) populations from which they are developedCitation11. The assessment of risk factors such as common SNPs, smoking and BMI are generally accepted as conferring high population attributable AMD risk but more genetic or environmental influencing factors and their distinct combination need to be identified in addition to their interactions to improve AMD prediction modelsCitation11. Moreover, while there is evidence that the risk of AMD and its progression to advanced (and blinding) forms can be modified by lifestyle changesCitation5,Citation7, it remains to be determined whether a change in the lifestyle of a person with early AMD delays or halts its progression. What is required are prospective, randomized studies that explore whether obese patients with early signs of AMD can slow their disease by losing weight or that smokers can delay AMD by stopping smoking and studies that provide a direct clinical quantification of these lifestyle adjustments.
Vision biomarkers in AMD
We argue that a better prediction of disease can only be achieved if we are able to quantify genetic and environmental factors in the causal mechanisms of AMD. While pathomechanisms in AMD are not completely understood, there is histologically a rod (night vision) over cone (day vision) photoreceptor vulnerability in response to the build up of pathological debris (drusen) within the layers (retinal pigment epithelium and Bruch’s membrane) underlying the photoreceptorsCitation12–13. An increased inflammatory/immune response due to a genetic risk variant coding for complement factor H (CFH) is thought to contribute to this build upCitation4 whereas for example environmental risk factors causing oxidative stress, infection, cardiovascular insufficiency and ischaemia may serve as triggersCitation14. With this knowledge, advancements in prediction of AMD onset may be made through development of direct and sensitive clinical (vision) tests that allow the measurement of the effects of environment and genetics on vision.
The influence of environmental risk factors such as diet on vision and its modifiable course may be determined using macular pigment densitometryCitation15. The macula is rich in pigments lutein (L) and zeaxanthin (Z) and studies support that the intake of L and Z rich diet may protect against the development of AMD. The relationship between macular pigment density and AMD is, however, unclear with some studies supporting that higher levels of macular pigment are associated with a lower risk of AMD and others showing no association between macular pigment and AMDCitation16–18. This may be in part due to the different technologies used for the assessment of macular pigment and the requirements for longitudinal studiesCitation19.
There is evidence for a polymorphic response of the visual system. Research on identical twins indicates that cone- and rod-mediated qualities of vision are not only determined by genetics but also largely by environmentCitation20. In particular, there is a strong genetic influence on cone-mediated flicker perception and strong environmental influences on adaptation dynamics and rod threshold adaptation kineticsCitation20. Vision tests that measure these particular qualities of vision could allow the possibility to test causal relationships between gene–environment interactions. Early studies demonstrate that there are sensitive vision tests such as dark adaptation and flicker perimetry that detect early vision deficits in patients with manifest AMD with normal (day) vision and that these visual qualities deteriorate with disease progressionCitation21–23. Promising recent advancements have been made, with mesopic (dim light) vision, where both cones and rods are active, being sensitive to sub-clinical vision loss in healthy persons with no AMD but with risk genotypes (CFH and ARMS2)Citation24. This study used techniques that applied flicker, cone and rod (mesopic) stimulation which have been associated with vision impairment in early AMD. The rationale behind applying these vision measures is based on evidence that ischaemia plays a key role in the pathomechanisms of AMD where reduced oxygenation of the neurosensory retina causes first visual function deficitsCitation14,Citation25–27. Both flicker stimulation and mesopic vision increase the oxygen demand making an eye susceptible to disease more prone to fail on these tests.
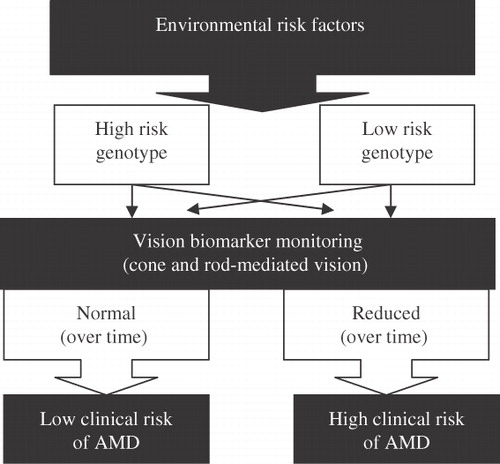
While the predictive value of genetic profiling alone is higher in AMD than most of the other multifactorial disease such as diabetes or coronary heart disease, complete causal mechanisms in AMD are far more complexCitation11. A comparison with disaster prediction has been made when it comes to multifactorial disease prediction suggesting that the likelihood of knowing all factors at a certain point of time to predict disaster (disease) is lowCitation11. A conceptual framework in outlines how a well defined vision test (that is sensitive to environmental and genetic interactions) could facilitate the prediction of disease. In this framework a healthy person with a low genetic risk profile but high environmental risk would show reduced visual function over time and therefore have a high clinical risk for developing AMD. In comparison a person with the same genetic profile and low environmental risk would have normal visual function at several time points and a low risk of developing AMD. In case of a high genetic risk, the vision biomarker would still account for the environment and depending on whether this risk is high or low, vision function would be reduced or normal over time.
Figure 1. Conceptual framework. Vision biomarkers determine clinical AMD risk (high or low) whereas genetic risk alone cannot. Each person has a unique exposure to environmental risk factors. For example in the case of a low risk genotype who develops AMD, vision biomarkers would show deterioration over time as environmental risk was higher. In case of a high risk genotype, vision biomarkers show no impairment over time as there is no (less) environmental risk. Thin arrows indicate alternate pathways.
Conclusion
In our enthusiasm for the discovery and early understanding of the genetics of AMD, some may advocate that genetic screening of AMD risk genotypes should be made available to the broader population; but what would this provide? Based on current knowledge there is limited clinical evidence available to provide appropriate guidance and advice on how to interpret the results. In those persons who are too young to express manifest disease, genetic testing may provide a risk estimate, allowing modification of lifestyle or diet to reduce the likelihood of disease. The advice could be to include a routine assessment of a vision test performed every two years and, based on follow up measurements, a clinical risk may be determined. In older patients with manifest AMD, genetics may inform treatment choices, since there are indications that genetic predisposition may account for the variability in response to treatmentCitation28. Prospective, randomized studies are needed to determine whether it is advantageous to change lifestyle or therapy based on a person’s genotype.
Whether a genetic test predicts a person has a high or a low risk of AMD, there is one inescapable fact: genetic screening of AMD does not detect disease, it simply provides a risk estimate with all the inaccuracies discussed above. Genetic studies have been instrumental in improving our understanding of the molecular pathology of AMD but when it comes to diagnosis, new clinical tests are needed that directly detect impending disease, preferably early, pre-clinical signs of disease. The first steps towards early detection of sub-clinical disease have been made suggesting sensitive rod and cone-mediated vision tests as candidate clinical vision biomarkersCitation21–22,Citation24. Long-term prospective studies are now needed to determine if these sensitive vision tests that detect sub-clinical vision impairment can predict manifest AMD.
Future research should focus on the further development of these clinical vision biomarkers to allow a rigorous assessment of the role of the interaction of environment and genetics and allow informed implementation of lifestyle interventions. Likewise, for some monogenic diseases where the genetic test can be replaced by direct measures of disease using biomarkers (e.g. salt content of sweat in cystic fibrosis), future technologies may move to a direct sensitive vision function test that can predict pre-clinical AMD and monitor its progression and the effect of lifestyle changes. In a multifactorial disease like AMD where many contributing genetic and environmental factors are still unknown, a vision test reflecting gene–environment interactions could add power to current statistical prediction models that are only based on known factors in selected populations.
Transparency
Declaration of funding
Supported by a Queensland University of Technology Vice Chancellor Fellowship Grant (BF) – the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of financial/other relationships
B.F. and C.P.M. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
Acknowledgements
We thank Dr Andrew J. Zele for review of a manuscript draft.
References
- Chakravarthy U, Evans J, Rosenfeld PJ. Age related macular degeneration. BMJ 2010;340:c981
- Access Economics. The global economic cost of visual impairment. 2010. Available at: http://www.amdalliance.org/user_files/documents/Global%20cost%20of%20VI_FINAL%20report.pdf [Last accessed 14 February 2011]
- Tong Y, Liao J, Zhang Y, et al. LOC387715/HTRA1 gene polymorphisms and susceptibility to age-related macular degeneration: a HuGE review and meta-analysis. Mol Vis 2010;16:1958-81
- Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. PNAS 2005;102:7227-32
- Schaumberg DA, Hankinson SE, Guo Q, et al. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch Ophthalmol 2007;125:55-62
- Chen Y, Zeng J, Zhao C, et al. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch Ophthalmol 2011;129:344-51
- Seddon JM, Francis PJ, George S, et al. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA 2007;297:1793-800
- Welsh MJ, Ramsey BW, Accurso F, et al. The metabolic and molecular bases of inherited diseases. Cystic fibrosis. In: Scriver C, Volgestein B, Beaudet AL, Sly WS, Valle D (eds.) New York: McGraw-Hill, 2001:5121-88
- Kraft P, Hunter DJ. Genetic risk prediction – are we there yet? N Engl J Med 2009;360:1701-3
- Hughes AE, Orr N, Patterson C, et al. Neovascular age-related macular degeneration risk based on CFH, LOC387715/HTRA1, and smoking. Invest Ophthalmol Vis Sci 2011;52:4694-702
- Janssens AC, van Duijn CM. Genome-based prediction of common diseases: advances and prospects. Hum Mol Genet 2008;17:R166-73
- Curcio CA. Photoreceptor topography in aging and age-related maculopathy. Eye 2001;15:376-83
- Guymer R, Luthert P, Bird AC. Changes in Bruch's membrane and related structures with age. Prog Ret Eye Res 1999;18:59-90
- Feigl B. Age-related maculopathy: linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Ret Eye Res 2009;28:63-86
- Wooten BR, Hammond BR Jr, Land RI, et al. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci 1999;40:2481-9
- Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case–Control Study Group. JAMA 1994;272:1413-20
- SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case–control study: AREDS Report No. 22. Arch Ophthalmol 2007;125:1225-32
- Dietzel M, Zeimer M, Heimes B, et al. Determinants of macular pigment optical density and its relation to age-related maculopathy: results from the muenster aging and retina study (Mars). Invest Ophthalmol Vis Sci 2011;52:3452-7
- Beatty S, van Kuijk FJ, Chakravarthy U. Macular pigment and age-related macular degeneration: longitudinal data and better techniques of measurement are needed. Invest Ophthalmol Vis Sci 2008;49:843-5
- Hogg RE, Dimitrov PN, Dirani M, et al. Gene–environment interactions and aging visual function: a classical twin study. Ophthalmology 2009;116:263-9
- Owsley C, Jackson GR, White M, et al. Delays in rod-mediated dark-adaptation in early age-related maculopathy. Ophthalmology 2001;108:1196-202
- Dimitrov PN, Guymer RH, Zele AJ, et al. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci 2008;49:55-65
- Phipps JA, Dang TM, Vingrys AJ, et al. Flicker perimetry losses in age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45:3355-60
- Feigl B, Cao D, Morris CP, Zele AJ. Persons with age-related maculopathy risk genotypes and clinically normal eyes have reduced mesopic vision. Invest Ophthalmol Vis Sci 2011;52:1145-50
- Grunwald JE, Hariprasad SM, DuPont J, et al. Foveal choroidal blood flow in age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci 1998;39:385-90
- Chen JC, Fitzke FW, Pauleikhoff D, et al. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci 1992;33:334-40
- Stefansson E, Geirsdottir A, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res 2011;30:72-80
- Kloeckener-Gruissem B, Barthelmes D, Labs S, et al. Genetic association with response to intravitreal ranibizumab (Lucentis) in neovascular AMD patients. Invest Ophthalmol Vis Sci 2011;52:4694-702
