Online Summary
Correction to: Rob Riemsma, Carol Forbes, Julie Harker, Gill Worthy, Kate Misso, Michael Schäfer, Jos Kleijnen and Steffen Stürzebecher. Systematic Review of tapentadol in chronic severe pain. Current Medical Research & Opinion 2011 Vol. 27, No. 10, 1907–1930.
Correction to: Rob Riemsma, Carol Forbes, Julie Harker, Gill Worthy, Kate Misso, Michael Schäfer, Jos Kleijnen and Steffen Stürzebecher. Systematic Review of tapentadol in chronic severe pain. Current Medical Research & Opinion 2011 Vol. 27, No. 10, 1907–1930.
The authors have been made aware of an error in . The results for Quality of Life in favour oxycodone when compared with tapentadol. This is not correct, it should favour tapentadol as shown in the corrected graph below. Although was correct, the legend was incomplete, this is corrected below.
Figure 6. (b) Tapentadol versus oxycodone for moderate to severe pain (continuous outcomes). * For Quality of Life we have reversed the result (MD = −0.06, instead of MD = 0.06) in order to be able to present the result of a positive outcome in the same graph as a negative outcome (such as Pain Intensity). The same applies to Quality of Sleep. SE = standard error; IV = inverse variance; CI = confidence interval; PGIC = Patient Global Impression of Change; Disc = discontinuations; AE = adverse events; SAE = Serious AE.
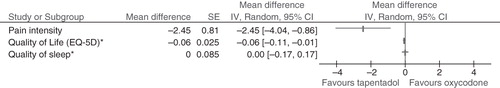
Figure 7. (b) Tapentadol versus placebo for moderate to severe pain (continuous outcomes). * For Quality of Life we have reversed the result (MD = −0.06, instead of MD = 0.06) in order to be able to present the result of a positive outcome in the same graph as a negative outcome (such as Pain Intensity). The same applies to Quality of Sleep. SE = standard error; IV = inverse variance; CI = confidence interval; PGIC = Patient Global Impression of Change; Disc = discontinuations; AE = adverse events; SAE = Serious AE.

This error did not affect the text or any of the study results or conclusions. The authors report the following revised and .
