Abstract
Asthma remains a formidable public health problem with ever increasing annual costs and prevalence. There are 300 million people with asthma worldwide. Per the Centers for Disease Control and Prevention, there are over 25 million Americans with asthma (both children and adults), i.e. one in 12 people have asthma, and this is increasing annually. Asthma results in approximately half a million hospitalizations and two million emergency department (ED) visits per year. In 2007 alone, 185 children and 3262 adults died from asthma, i.e. nine to ten patients die a day from asthma. This resulted in an annual cost of $56 billion in medical costs, lost work/school days, and early deaths. Therefore, we need novel and innovative therapies for asthma.
In this Editorial, I review results from a study by Tse et al. evaluating the therapeutic potential of statins, within the context of our current state of knowledge. I review observational studies and clinical trials, highlight some potential pitfalls in clinical trial design, and discuss important questions for future research.
Statin use and asthma outcomes: findings, limitations, and opportunities
Tse et al.Citation1 report a large retrospective cohort observational study evaluating whether statin use in asthmatics was associated with decreased asthma-related hospitalizations and/or ED visits. A population-based administrative database was used to determine outcomes in adults on statins over a period of 12 months. Using pharmacy records, the authors defined statin use based on a measure of ‘statin exposure’. Patients were stratified into two groups according to their inhaled corticosteroid (ICS) use, a total of 3747 ICS users and 2905 non-ICS users. The authors found that among ICS users, statin use was associated with reduced odds of asthma-related ED visits (OR = 0.77, 95% CI 0.64–0.94, p = 0.008), but not with asthma-related hospitalizations. Non-ICS users showed no significant associations.
The strengths of this study include a large real-life cohort of asthmatics, an a priori evaluation based on ICS use, and an assessment of statin exposure up to the time of asthma-related events. This work provides indirect evidence that statins may improve important asthma outcomes such as acute exacerbations. This work along with othersCitation2–4 provides support for a large randomized controlled trial (RCT) of a statin intervention in asthma.
As with any observational study, there are unavoidable limitations. Although the authors attempted to control for residual confounding by statin indication in their multivariate model (e.g. ischemic heart disease, stroke, diabetes), this does not fully account for the fact that the two populations are different hosts. However, even though statin users were a sicker cohort, they still did better than non-users indicating that statin use may have had a beneficial effect. Of note, lung function as a measure of baseline asthma severity, such as FEV1% predicted, was not available and therefore not accounted for in their statistical models. The authors also did not exclude patients with overlapping asthma and COPD whose response to statins may be different. Finally, a common criticism of statin observational studies is the confounding effect of the ‘healthy user effect’; however, others have attempted to account for this phenomenon and still showed a benefit to statin useCitation5. To know with greater confidence, properly designed multi-center RCTs are needed.
The reason(s) for the discrepancy between asthma-related ED visits and hospitalizations in Tse et al.’s study is not clear. Statin use was associated with an improvement in the former but not the latter. Tse et al.’s findings regarding asthma-related hospitalizations differs from that of other authorsCitation2,Citation3. While many reasons can be hypothesized to explain this discrepancy, one can presume that a combination of different factors led to an ED visit rather than hospitalization. Patients who were hospitalized due to their asthma may have more severe disease and, thus, it becomes important to carefully consider the hospitalized patient in future studies.
However, despite these limitations, observational studies including this one by Tse et al. do point us in a new direction. It provides important data to inform the planning of future asthma–statin RCTs. These data suggest that statins should be given to asthmatics already on ICS and for at least 12 months. Data already exist to support the additive or synergistic effects of statins and ICS in human asthmaCitation6,Citation7. The beneficial effects of statins in reducing sputum inflammatory markersCitation8, yet lack of improvement in lung function, could be due to the short treatment duration (i.e. maximum 8 weeks long) of statin therapyCitation8,Citation9. The authors acknowledge that it may take years before positive effects on lung function and/or airway remodeling may be seen with statins.
Published randomized controlled trials in asthma using statins
Over the past six years or so, interest in the topic of statins as a potential therapy for airway disease such as asthma and COPD has grown. Investigators from varied countries and disciplines have evaluated the therapeutic potential of statins. However, most of these studies have been observational in natureCitation2–5, and the handful of RCTs performed have yielded mixed results at bestCitation7,Citation10–15. In addition, none of the clinical trials evaluated important endpoints such as acute exacerbations or ED/hospital admissions. Despite this, some benefits were noted in two of the seven reported trials. Braganza et al. showed a benefit in smoking asthmatics with improved quality of life (QOL) scoresCitation11, while Cowan et al. reported improved ACQ (Asthma Control Questionnaire) asthma control scores, FEV1, and sputum eosinophil counts in mild asthmatics weaned off of ICSCitation12. While statins cannot yet be recommended for the treatment of asthma in the absence of stronger results from RCTs, significant opportunities remain as this area is ripe for investigations on several levels.
It is well accepted that the statins have revolutionized the treatment of cardiovascular diseases. In addition to reducing cardiovascular events and deaths due to myocardial infarctionsCitation16, statins amazingly reverse atherosclerotic vascular wall remodelingCitation17 thereby improving coronary blood flow. This key observation is not seen over a scale of weeks or months, but rather over years of treatment. Therefore, the temporal relationship between statin use and the asthma outcome of interest becomes a very important point of consideration. Might the same thing be possible in asthmatic airways with respect to adverse structural remodeling and luminal narrowing? This is perhaps the single most important point to be raised regarding the lack of efficacy in the aforementioned asthma–statin RCTs – the treatment duration. The above RCTs studied a statin intervention over 4 or 8 weeks. Based on Tse et al.’s findings and that of others, we may need 1 to 2 years of statin treatment before acute exacerbations and/or oral corticosteroid use are reduced; and perhaps longer duration for anti-remodeling effects.
Potential benefits in asthma
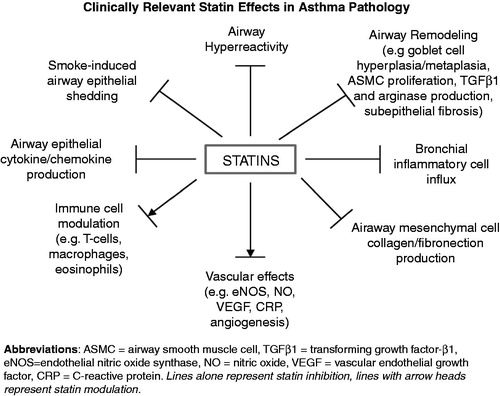
Given the biological plausibility of the statins with respect to lung and airway diseases in particular, and given their pleiotropic immunomodulatory effectsCitation18,Citation19, it is reasonable to think that the statins could have a beneficial effect in asthma (). This would require the correct experimental conditions, the right type of statin, and the appropriate patient population that is most likely to benefit.
Many additional areas of investigation are needed in order to better understand statins’ potential benefits and/or potential harms. To date, we still do not know whether an orally ingested statin even reaches the airway compartment, let alone the bronchial epithelium – but we assume it does. The answer to this important question has clear implications for direct statin effects on airway resident cells such as epithelial, mesenchymal (e.g. fibroblasts, airway smooth muscle), and endothelial cells, including the resident immune cells (e.g. macrophages and airway dendritic cells).
The immune and endothelial effects of statins are generally well established in the cardiovascular literature. Some work has already been done in epithelial and mesenchymal cells, but we are in the early stages. For instance, simvastatin inhibits airway smooth muscle cell proliferation in vitroCitation20 and airway hyperreactivity in vivoCitation21,Citation22, while inducing apoptosis in airway mesenchymal cellsCitation23,Citation24. Might statins mitigate airway smooth muscle cell mass and thereby reduce airflow obstruction? Statins also have differential effects on epithelial cytokine production, with most publications indicating inhibition of pro-inflammatory cytokinesCitation25–28. Furthermore, detailed mechanistic studies are also needed in order to elucidate the underlying mechanisms involved. Thus, the central question of direct statin effects on airway structural cells in the human host, and the logical link to route of administration and drug development, remain critical areas for research.
Questions for future clinical studies
As one contemplates the next steps in research, the following questions arise:
What is the ideal route of administration, i.e. oral versus inhaled versus both?
What are statins’ effects on airway epithelial mucosal immunity and barrier integrity?
Which combination treatments (ICS, LABA, LAMA, LTRA, etc. ± statin) might have the best effect on reducing asthma exacerbations and airway remodeling/disease progression, while improving lung function?
Which class of statin has the greatest potential for benefiting asthmatics (i.e. hydrophilic versus hydrophobic statins)?
What about the pediatric asthma population? When is it too early (or too late) to begin treatment? Is it safe to use a statin in this population?
What lung-specific versus systemic biomarkers (or gene signatures) might predict a ‘statin-responsive’ asthmatic subgroup?
What asthma phenotype or sub-phenotype is most likely to benefit?
Many more questions exist and will likely arise. While tempered optimism is appropriate regarding statins in asthma, given the widespread use of statins worldwide, serious efforts should be undertaken to answer the above questions. Beyond their fascinating biological activities, the statins have the potential to be an important adjunctive therapy in our treatment armamentarium. Advances in this area might usher in the next innovative yet hopefully inexpensive therapy for asthma.
Transparency
Declaration of funding
This editorial was not funded.
Declaration of financial/other relationships
A.A.Z. has disclosed that he has no significant relationships with or financial interests in any commercial companies related to this study or article.
References
- Tse SM, Charland SL, Stanek E, et al. Statin use in asthmatics on inhaled corticosteroids is associated with decreased risk of emergency department visits. Curr Med Res Opin 2014;30:685-93
- Huang CC, Chan WL, Chen YC, et al. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Investig 2011;41:507-12
- Lokhandwala T, West-Strum D, Banahan BF, et al. Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective cohort analysis. BMJ 2012;2:1-8
- Zeki AA, Oldham J, Wilson M, et al. Statin use and asthma control in patients with severe asthma. BMJ Open 2013;3:1-10
- Alexeeff SE, Litonjua AA, Sparrow D, et al. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med 2007;176:742-7
- Maneechotesuwan K, Kasetsinsombat K, Wamanuttajinda V, et al. Statins enhance the effects of corticosteroids on the balance between regulatory T cells and Th17 cells. Clin Exp Allergy 2013;43:212-22
- Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, et al. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol 2010;126:754-62.e1
- Yuan C, Zhou L, Cheng J, et al. Statins as potential therapeutic drug for asthma? Respir Res 2012;13:108
- Si X, Zhang S, Huo L, et al. Statin therapy does not improve lung function in asthma: a meta-analysis of randomized controlled trials. J Int Med Res 2013;41:276-83
- Hothersall EJ, Chaudhuri R, McSharry C, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax 2008;63:1070-5
- Braganza G, Chaudhuri R, McSharry C, et al. Effects of short-term treatment with atorvastatin in smokers with asthma – a randomized controlled trial. BMC Pulmonary Medicine 2011;11:16
- Cowan DC, Cowan JO, Palmay R, et al. Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax 2010;65:891-6
- Menzies D, Nair A, Meldrum KT, et al. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol 2007;119:328-35
- Fahimi F, Salamzadeh J, Jamaati H, et al. Do statins improve lung function in asthmatic patients? A randomized and double-blind trial. Iranian J Pharmaceut Sci 2009;5:13-20
- Moini A, Azimi G, Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: a double-blind randomized clinical trial. Allergy Asthma Immunol Res 2012;4:290-4
- Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 2008;52:1769-81
- Nicholls SJ, Grasso AW, Schoenhagen P, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007;297:499-508
- Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006;6:358-70
- Liao J, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005;45:89-118
- Takeda N, Kondo M, Ito S, et al. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol 2006;35:722-9
- Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med 2009;180:731-40
- Ahmad T, Mabalirajan U, Sharma A, et al. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. Am J Respir Cell Mol Biol 2011;44:531-9
- Ghavami S, Mutawe MM, Sharma P, et al. Mevalonate cascade regulation of airway mesenchymal cell autophagy and apoptosis: a dual role for p53. PloS One 2011;6:e16523
- Ghavami S, Mutawe MM, Hauff K, et al. Statin-triggered cell death in primary human lung mesenchymal cells involves p53-PUMA and release of Smac and Omi but not cytochrome c. Biochim Biophys Acta 2010;1803:452-67
- Iwata A, Shirai R, Ishii H, et al. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol 2012;168:234-40
- Sakoda K, Yamamoto M, Negishi Y, et al. Simvastatin decreases IL-6 and IL-8 production in epithelial cells. J Dent Res 2006;85:520-3
- Murphy DM, Forrest IA, Corris PA, et al. Simvastatin attenuates release of neutrophilic and remodeling factors from primary bronchial epithelial cells derived from stable lung transplant recipients. Am J Physiol Lung Cell Mol Physiol 2008;294:592-9
- Zeki AA, Thai P, Kenyon NJ, Wu R. Differential effects of simvastatin on IL-13-induced cytokine gene expression in primary mouse tracheal epithelial cells. Respir Res 2012;13:38