Abstract
Objectives:
Flu-like symptoms (FLS) are commonly experienced by patients receiving interferon gamma-1b which may cause discontinuation or disruption of dosing during initial therapy or on re-initiation following a break in therapy. In contrast to Type I interferons, the impact of dose-titration on FLS has not been reported and is not a practice described or included in the approved prescribing information for interferon gamma-1b.The objective of this study was to assess the effect of a 2 week titration regimen on the severity of FLS during the initial 3 weeks of therapy with three times weekly subcutaneous injections of interferon gamma-1b.
Methods:
Healthy men and women were randomized into a double-blind, two-period, crossover study. Each study period was 3 weeks in duration and there was a minimum 15 day washout between treatment periods. Two treatment regimens were compared: No Titration dosing (full 50 mcg/m2 subcutaneously [SC] three times weekly for 3 weeks) and Titration (15 mcg/m2 SC three times weekly during week 1, 30 mcg/m2 SC three times weekly during week 2 followed by the full dose of 50 mcg/m2 SC three times weekly during week 3). Subjects remained in the clinic for at least 12 hours following each injection. FLS was based on a composite score for fever, chills, tiredness and muscle aches assessed at baseline and 4, 8 and 12 hours following each injection. Acetaminophen was allowed at the discretion of the PI. The primary endpoint was the change from baseline in FLS severity at 8 hours averaged over the 3 weeks of treatment. Additional endpoints included FLS at 4 and 12 hours, individual flu-like symptoms, rates of discontinuation, incidence of FLS and acetaminophen use.
Clinical trials registration:
NCT 01929382.
Results:
Of the 40 subjects randomized, there were 15 (37.5%) discontinuations. Titration resulted in a significant reduction in FLS severity at 8 hours (p = 0.023) averaged over the 3 week treatment period. The difference in 3 week FLS severity reflects differences during week 1 treatment, indicating an early peak in FLS severity during the No Titration treatment and subsequent development of tolerance. In contrast, titration results in near baseline severity scores throughout the treatment period. Similar trends were seen for 4 and 12 hour FLS severity scores. Of the individual FLS, difference in fever severity was most marked. Safety profiles for both regimens were consistent with the approved prescribing information for interferon gamma-1b. Study limitations included the use of healthy subjects rather than disease subjects, the lack of a validated assessment tool for evaluating FLS and the relatively high discontinuation rate.
Conclusion:
A short 2 week, dose-titration regimen reduces FLS severity following interferon gamma-1b treatment initiation in normal subjects.
Introduction
Flu-like symptoms (FLS) are commonly experienced with interferon therapyCitation1. In the case of the Type I interferon beta approved for use in multiple sclerosis, tolerance or tachyphylaxis usually develops after a number of weeks of therapy and therefore a gradual or fractionated dose introduction for these products has been widely adopted to reduce the severity and/or incidence of FLS after initiating therapyCitation2. However, there is no consistency to the number of dose adjustment steps, the dose increments, the overall duration of the titration period or the total number of titration injections between the three approved product regimens. A titration regimen based on more than a single-step dose adjustment appeared more effectiveCitation3,Citation4. More recently, a comparison in healthy subjects on a multi-step regimen based on a shorter 3 week titration duration appeared superior to a longer 6 week duration in reducing FLS severity and incidence following weekly intramuscular injection of interferon beta-1aCitation5.
Interferon-gamma-1b (Actimmune), a recombinant form of human interferon gamma, is classified as a Type II interferon. It is approved for reducing the frequency and severity of serious infections associated with Chronic Granulomatous Disease (CGD) and additionally, in the USA, for delaying time to disease progression in patients with severe, malignant osteopetrosis (SMO). Both these conditions are of low prevalence (1:200,000 for CGDCitation6 and 1:250,000 for SMOCitation7) and are life-threatening rare diseases. Interferon gamma-1b is administered three times weekly by subcutaneous (SC) injection. Interferon gamma-1b is associated with a profile of FLS side effectsCitation8. However, there is no established practice or instruction relating to an initial dose titration of interferon gamma-1b nor has there been any specific report on the profile of FLS following its administration, or the impact of dose titration following initial therapy. In proposing a titration regimen for interferon gamma-1b, a key consideration is to minimize the overall duration of reduced exposure in order to limit any potential risk of serious infection (in CGD) or disease progression (in SMO). Because of the small size of population of both children and adults with these disorders, investigations into interferon gamma-1b related FLS and impact of titration regimens are not feasible in patients. Therefore, the effects of a short 2 step, 2 week titration in reducing FLS severity was assessed in this study in healthy subjects.
Subjects and methods
Study sample
Healthy male and female subjects (18–55 years old), capable of understanding the purpose of the study and providing informed consent, with body mass index (BMI) of 18–32 kg/m2 and body weight range of 50 kg to 120 kg, were eligible to participate in this study (NCT 01929382). Female subjects of childbearing potential were required to practice effective contraception during the study and to continue contraception for 30 days after the last dose of study treatment. Subjects were excluded if: they had a known history of, or positive test result for, human immunodeficiency virus (HIV), hepatitis C virus, or hepatitis B surface antigen (HBsAg), or a known history of chronic fatigue syndrome or fibromyalgia, or a history of significant depression or other mood disorder. Subjects were excluded if they had experienced flu-like illnesses (e.g., gastroenteritis, upper respiratory infection, common cold) within 1 month, had a history of premalignant and/or malignant disease, a history of severe allergic reactions to any drug or any history of anaphylactic reactions or if they had a known allergy to interferon gamma-1b or any of its components. Subjects were excluded if they had a history or evidence at screening of any alcohol or substance abuse or any clinically significant cardiac, endocrinologic, hematologic, hepatic, immunologic, metabolic, urologic, pulmonary, neurologic, dermatologic, psychiatric, renal, or other major disease. Other reasons for exclusion included any serious infection (e.g., pneumonia, septicemia) within the 3 months prior to screening or active bacterial or viral infection at screening or any history of alcohol or substance abuse. Also excluded were any female subjects who were pregnant or breastfeeding, any subjects with clinically significant abnormal electrocardiogram (ECG), participation in any other investigational drug study within 4 weeks prior to Day 1 or within five half-lives of the previous investigational treatment, whichever was longer, use of any prescription products within 4 week(s) prior to Day 1 with the exception of contraceptives or any topical dermatology applications to treat skin diseases (except Retin A). Non-prescription medications containing acetaminophen or non-steroidal anti-inflammatory medications within 24 hours prior to Day 1 were also prohibited as were allergy shots or desensitization therapy within 1 month prior to Day 1, vaccinations within 2 weeks prior to Day 1, blood donation greater than 480 mL within 30 days prior to screening, use of any tobacco product more than five times within 30 days prior to screening or any use within 2 days prior to Day 1 of both treatment periods, or unable or unwilling to comply with study requirements.
The study was conducted at Prism Research (St Paul, MN, USA) in accordance with national and local laws and regulations, the International Conference on Harmonisation guidelines for Good Clinical Practice, as well as the Helsinki Declaration (2008 revision). The study protocol was reviewed and approved by an independent review board (IRB); RCRC Independent Review Board LLC. All subjects gave written informed consent before being evaluated for eligibility.
Study design
The study drug was commercial interferon gamma-1b which is a sterile, clear, colorless solution filled in a single-use vial for subcutaneous (SC) injection. Each 0.5 mL contains 100 mcg (2 million IU) of interferon gamma-1b formulated in 20 mg mannitol, 0.37 mg disodium succinate hexahydrate, 0.14 mg succinic acid, 0.05 mg polysorbate 20 and sterile water for injection.
This was a randomized, double-blind, crossover study in 40 healthy adult volunteers. Eligible subjects were randomized in a 1:1 ratio to a sequence to receive Treatment A followed by Treatment B, or Treatment B followed by Treatment A, in a crossover fashion. In ‘Treatment A: No Titration’, subjects received the recommended dose of interferon gamma-1b by SC injection three times weekly from week 1 through week 3. In ‘Treatment B: Titration’, subjects were titrated up to the recommended dose of interferon gamma-1b over a 3 week period. Subjects received 30% of the recommended dose during week 1, 60% of the recommended dose during week 2, and the recommended dose during week 3. There was a minimum 15 day washout period between dosing periods.
All interferon gamma-1b doses were administered in the clinic. Subjects remained in the clinic for observation for at least 12 hours following each dose. On days of dosing, subjects were evaluated for baseline flu-like symptoms (FLS) and injection site reactions (ISR) within 1 hour prior to dosing and then at 4, 8 and 12 hours following each dose. Subjects returned to the clinic 10 to 14 days after their last dose for a final safety evaluation. An unblinded pharmacist at the study center prepared each injection in accordance with the randomization schedule. The injection volume varied with titration dose. To maintain the blind, an unblinded study nurse or designee administered the injection in a manner such that neither the subject nor other study personnel could see the volume of interferon gamma-1b in the syringe. All other study personnel and the sponsor were blinded to treatment assignments, with the exception of an unblinded statistician who was responsible for generating the randomization list.
Assessments
FLS was defined as the presence of fever (as measured by oral body temperature), chills, muscle aches and tiredness. Subjects were assessed for FLS within 1 hour before each interferon gamma-1b injection, and again at 4, 8 and 12 hours following each injection. Each symptom was evaluated on a 4 point severity scale as shown in .
Table 1. Criteria for assessment of FLS.
Discontinuation rate was defined as subjects who did not complete the study per protocol (i.e., both treatment periods).
ISRs were defined as the presence of erythema, induration, tenderness or temperature at the site of interferon gamma-1b injection. Subjects were assessed for ISR within 1 hour before each interferon gamma-1b injection and again at 4, 8 and 12 hours following each injection. The severity of erythema, induration, and tenderness was assigned a score of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). Severity of temperature was assigned a score of 0 to 2 (0 = normal, 1 = warm, 2 = hot).
Subjects were assessed for the presence of headache, nausea and vomiting within 1 hour before each interferon gamma-1b injection and again at 4, 8, and 12 hours following each injection. Severity of each symptom was assigned a score of 0 to 4, with definitions following those for FLS ().
Each dose of acetaminophen taken by a subject at any time during the study was recorded by site personnel.
Adverse events (AEs) were monitored continuously during the study, starting immediately after the first dose of study drug. Subjects were instructed to report all AEs experienced during the study, and subjects were assessed for the occurrence of AEs throughout the study. A comprehensive physical examination was performed at screening and at the end of the study, 10 to 14 days after the last injection. A targeted physical assessment (a symptom-directed evaluation) was performed at the Day 1, 8 and 15 visits for both treatment periods. Vital sign measurements (blood pressure, pulse rate, respiration rate) were collected prior to each injection. Oral body temperature was obtained prior to each injection and at the time of each FLS assessment. A 12-lead ECG reading and samples of blood and urine for analysis of hematology, serum chemistry and urinalysis were collected at screening and at the end of the study, 10 to 14 days after the last injection.
AEs were summarized by treatment regimen. AEs with onset after the start of study drug were summarized and grouped by MedDRA System Organ Class (SOC) and specific AE. Results were displayed in order of decreasing frequency, both across SOC and within each SOC term. In addition, AEs were recorded by severity (mild, moderate, severe) and by relationship to study drug (Definite, Possible, Not Related). ‘Definite’ and ‘Possible’ are grouped as ‘Related’.
Statistical analyses
Analysis methods
Analyses were prospectively described in a statistical analysis plan. All analyses were performed using SAS Version 9.2 statistical software (SAS Institute, Cary, NC, USA). All statistical tests were two-sided and at α ≤0.05 level of significance. Missing data were not estimated or carried forward in any of the analyses, except for the sensitivity analysis of the primary endpoint. All subjects who were randomized to a treatment sequence and received at least one dose of study treatment were used for all efficacy and safety analyses.
The primary endpoint was defined as the change in severity of FLS from baseline (pre-injection) at the 8 hour post-injection time point over the 3 weeks of therapy/injections. Due to the repeated measures within each injection day and across the three treatment weeks, the change from baseline FLS scores was analyzed using the linear mixed model for a two-treatment, two-period crossover trial modified for repeated measures. The model included factors for treatment, period and sequence. Because subjects discontinued the study during a treatment period, all data up to the point of discontinuation were used in the primary analysis. To assess for sensitivity to discontinuation, the last observation of FLS was carried forward to the last assessment period for discontinued subjects. In addition, covariates were used to identify whether or not the subject discontinued and to quantify the number of study days from first injection to discontinuation.
Because a single primary endpoint was defined prospectively, no adjustments for multiplicity beyond the repeated measures approach were required. Time was defined as injection days and treatment weeks. Secondary endpoints were analyzed for exploratory purposes and thus were not adjusted for multiplicity.
Discontinuation rates, after the first period, for the two treatment regimens were performed using a two-sided Fisher’s Exact Test. The statistical analysis was limited to the first period to minimize bias introduced from discontinuations in the second period.
Additional efficacy analyses included change in severity of FLS and in each component of FLS (chills, fever, muscle aches and tiredness) from baseline (pre-injection) at the 4, 8 and 12 hour post-injection time point over the 3 weeks of therapy/injections and by week of therapy. All analyses of change in FLS severity at different time points post-treatment were analyzed using the same type of linear mixed model as specified for the primary analysis without the sensitivity for discontinuations.
Incidence of FLS (≥2 point FLS score change post- versus pre-injection) was assessed at the 4, 8 and 12 hour post-injection time points averaged over the 3 weeks of therapy/injections. All incidences of FLS post-treatment were analyzed using generalized estimation equations with repeated measures for each week.
Severity of ISR, severity of overall impact on functioning and severity of headache, nausea and vomiting at each post-injection time point (4, 8 and 12 hour) were based on the average score over the 3 weeks of treatment/injections and were analyzed using Cochran–Mantel–Haenszel test by injection day and time point. Incidence of headaches, nausea, vomiting and acetaminophen use was analyzed by Fisher’s Exact two-sided test by time and day.
Sample size
Based on a similar studyCitation5 of FLS severity in healthy subjects with titration of interferon beta 1-a, the change from baseline FLS of a No Titration regimen was expected to be 0.54 at peak post-injection, whereas the Titration regimen was expected to have a change of 0.13. The standard deviation (SD) of the differences was expected to be 0.76 and, therefore, 20 subjects per sequence were estimated as sufficient to detect statistically significant treatment differences at α = 0.05 with a power of 90%.
Results
Subjects
A total of 40 healthy adult subjects were enrolled and randomized to treatment sequence. A total of 15 subjects discontinued the study prematurely, including 4 subjects who experienced FLS or AEs (). Thus, the number of subjects in each treatment sequence were 20 and 20 respectively but due to discontinuations before period 2 the number of subjects by treatment were 32 (Titration) and 35 (No Titration) ().
Table 2. Subject disposition by treatment sequence.
Table 3. Discontinuation rates by treatment regimen and treatment period.
When evaluated for the first treatment period only, the proportion of subjects who discontinued from the Titration regimen was 25% compared to 40% in the No Titration regimen. However, these were not statistically significantly different ().
Demographics and baseline characteristics were similar across treatment sequence (). Ages ranged from 18 to 54 years. The distribution between women (n = 22) and men (n = 18) was balanced overall, and by treatment sequence. The mean BMI (25.6) and BSA (1.88) were also balanced overall, and by treatment sequence.
Table 4. Demographics and baseline characteristics by treatment sequence.
According to the study protocol, nine injections of interferon gamma-1b were to be administered during each treatment period, for a total of 18 injections. Twenty-six (81%) subjects, of the 32 subjects who participated in the Titration regimen, received all nine injections. Twenty-seven (77%) subjects, of the 35 subjects who participated in the No Titration regimen, received all nine injections.
FLS severity
The Titration regimen was associated with a lower FLS severity score at 8 hours post-injection over 3 weeks (the pre-specified primary endpoint) compared with the No Titration regimen and when adjusted for drop-out and treatment length (sensitivity analysis), this effect reached statistical significance at p = 0.02 (). There was no statistically significant study period or treatment sequence effect (). Analysis by individual day showed a statistically significant difference on Day 1 ().
Figure 1. Mean change in severity of FLS at 8 hours post-injection over 19 days, by treatment regimen.
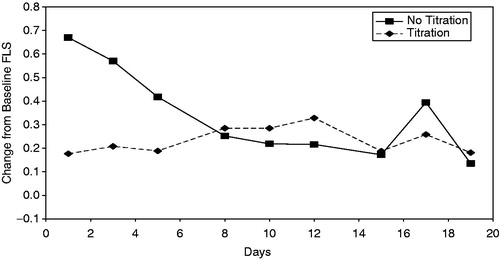
Table 5. Change in severity of FLS scores at 8 hours post-injection over 3 weeks, by day.
The FLS data were also analyzed based on 4 and 12 hour post-injection severity scores and by day and week. The Titration regimen showed a statistically significant lower severity of FLS during week 1 at 4, 8 and 12 hours.
Additional analysis
When each component of 8 hour FLS was analyzed, only fever () showed statistically significant lower severity, overall 3 week (p < 0.01) and for week 1 (p < 0.0001). This was similar for fever at 12 hours post-injection during week 1 (p < 0.0001), but not at 4 hours post-injection during week 1. Severity of chills and tiredness were less severe for the Titration regimen compared to the No Titration regimen at the 4 hour post-injection time point during week 1 (p = 0.01 and p = 0.03 respectively).
Figure 2. Mean change in severity of fever at 8 hours post-injection over 19 days, by treatment regimen.
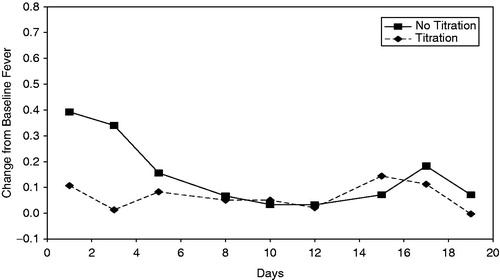
Analysis for the incidence of 8 hour FLS score ≥ 2 was significantly lower for the Titration regimen at week 1 (p = 0.02) with an odds ratio of 0.02. This was also the case for FLS incidence at 4 hours post-injection for week 1 (p = 0.04) with an odds ratio of 0.06. No subject had an FLS score ≥2 at the 12 hour post-injection time point.
Consistent with prescribing information, the use of acetaminophen was allowed at the discretion of the investigator. Overall there was a trend towards lower acetaminophen use during the Titration regimen (34%) compared with the No Titration regimen (46%), though not statistically significant. These trends were most marked on Day 1 (6% versus 23%) and Day 2 (3% versus 21%) and statistically significant on Day 5 (0% versus 19%, p = 0.02).
Though there was a trend for better overall function (i.e., more mild than moderate scores) for the Titration regimen compared to the No Titration regimen, the differences did not reach statistical significance except at the Day 1, 4 hour time point (p = 0.03). There was a trend for less headache in the Titration regimen compared to the No Titration regimen, but the difference did not reach statistical significance. No subjects experienced vomiting and the overall incidence of nausea was low (≤2 subjects) for any regimen.
The overall incidence and severity of ISR were low and mild. However, subjects in the Titration regimen had less severe erythema on Day 1 (p = 0.01) and Day 3 (p = 0.02) compared to the No Titration regimen.
Safety and tolerability
Adverse Event (AE) reporting began at the time of the first injection of study drug and continued through the last study visit. AE reporting included FLS, ISR and headache, nausea and vomiting which were also analyzed separately as efficacy endpoints. Almost all subjects experienced at least 1 AE, regardless of treatment regimen; 97% in the Titration group compared to 91% in the No Titration group. The number of subjects with an AE considered related to the study drug was also similar across treatment regimen (94% Titration, 91% No Titration).
Four subjects in the No Titration group experienced severe AEs considered to be related to study drug, compared to none in the Titration group. The AEs were neutropenia, elevated white blood cell (WBC) count, flu-like symptoms and traumatic brain injury with loss of consciousness (also a Serious Adverse Event [SAE]).
No deaths occurred during the study. There was 1 SAE reported by 1 subject, and, although the investigator considered the event possibly related to study drug, the subject was subsequently lost to follow-up despite multiple attempts by the site to contact him.
summarizes all AEs occurring in >5% of subjects by treatment regimen and by system organ class in descending frequency. The system organ class with the most AEs for both treatment regimens was general disorders and administration site conditions (88% Titration, 83% No Titration). This category captures the majority of FLS and ISR. The next most AEs across both treatment regimens included headache, nausea, and myalgia, again either an AE of interest or FLS.
Table 6. Summary of adverse events by system organ class occurring >5% by treatment regimen.
Discussion
The results of this study confirm that interferon gamma-1b administration in healthy subjects is associated with a profile of FLS similar to that reported previously for interferon gamma-1b in the approved CGD patient populationCitation8. This study also demonstrates that a titration regimen involving a two-step, 2 week dose escalation significantly reduced the severity of FLS following initiation of therapy in healthy subjects.
The impact of FLS on adherence to interferon gamma-1b therapy has been described in a long term (up to 9 year follow up), open label study in 76 CGD patients receiving interferon gamma-1b by Marciano et al.Citation9. Marciano et al.Citation9 reported a therapy interruption (<2 months) rate of 13% primarily related to FLS type side effects. Given the established utility of interferon gamma-1b, as part of prophylactic therapy, in CGD patients to reduce the frequency of serious infections, a potential dosing strategy to minimize FLS on therapy initiation or re-initiation following interruption may have important implications for its optimal clinical use. This study was conducted in healthy subjects and its applicability to the target patient population is not established. It would certainly be desirable to minimize the duration of any titration period since a reduced interferon gamma-1b exposure may increase the risk of serious infection. Any clinical adoption of the titration regimen would need to consider possible increased risk related to short-term underexposure to interferon gamma-1b with potential benefits of improved adherence by facilitating therapy initiation or re-initiation following treatment interruption. Alternatively, the results of this study offer some guidance as to the relatively short period of peak FLS severity with standard dosing which may be acceptable to some patients.
FLS is a syndrome of common ‘constitutional’ symptoms, yet there is no standard or clinically validated assessment instrument to measure FLS severity. In the case of interferon beta-1a, the recent report by Matson et al.Citation5, also in healthy subjects, focused on a composite FLS score based on four core symptoms, namely fever, chills, fatigue and muscle aches. This measure appeared to be sensitive to the incidence and severity of FLS associated with weekly intramuscular injections of interferon beta-1a, the time course of development of tolerance to FLS after repeated administrations and to the ability of titration regimens to minimize the severity and incidence of FLS on initiation of therapy. The current study used a similar methodology to assess FLS severity following initial administration with interferon gamma-1b. Indeed, the pattern of FLS severity was similar to that reported by Matson et al.Citation5; thus, an initial peak composite FLS severity score was observed on first administration of interferon gamma-1b with a subsequent reduction in severity after repeated administrations and a titration associated reduction in FLS severity that eliminated the higher initial FLS severity. This suggests a potential general utility to this particular FLS measurement instrument that could be applied to other interferons and potentially more widely in characterizing both FLS and the impact of titration regimens due to other exogenous cytokines or other FLS-inducing agents. There were some important differences in study design from that reported by Matson et al.Citation5, including the conduct of this study as a crossover design, which allowed a more efficient sample size and minimized the need for a large numbers of healthy subjects to be exposed to interferon gamma-1b. While a relatively short washout period (>15 days) between study periods was used, there was no statistically significant study period or treatment sequence effect seen in the primary endpoint analysis. Additionally, in contrast with Matson et al.Citation5, which prescribed the use of acetaminophen prior to and three times following each injection regardless of need or utility; in this study, the use of acetaminophen was allowed but at the discretion of the principal investigator. In this more naturalistic setting, about 50% of subjects received acetaminophen in the No Titration phase and there was a non-significant trend toward reduced acetaminophen use in the Titration phase.
The success of dose escalation titration in reducing initial FLS severity depends on the development of tolerance to FLS on continued administration. Thus, in the No Titration regimen, the peak FLS severity is observed following first administration and the severity reduces progressively on subsequent administrations until by week 2 the severity is re-set close to baseline. In contrast, the lower initial doses of interferon gamma-1b in the titration regimen do not have this initial peak FLS effect and, by inducing tolerance during the dose escalation periods, allows full dosing in week 3 without any spike in FLS severity. This tolerance phenomenon is also observed with the interferon beta therapies, albeit the time course of development of tolerance appears longer compared with our results. In the case of Matson et al.Citation5, this involved 3 to 4 weeks of weekly intramuscular injections. It is not clear what the reason for this difference is, but could reflect differences in dosage interval, differences in pharmacokinetics or pharmacodynamics between the different interferons. A common feature of both studies is the number of injections associated with the induction of tolerance which is similar (3–4 injections) between both titration regimens and may suggest this as a critical variable in designing titration regimens for other cytokines. The mechanism of the development of tolerance to acute FLS is unclear. Certainly, the phenomenon of pyrogenic tolerance is well known and has been described for exogenously administered pyrogenic cytokines including interferon gammaCitation10,Citation11. However, the exact mechanisms remain to be determined.
Of all the FLS symptoms, fever is the most consistently observed and, in the case of interferon gamma-1b, is the highest frequency reported side-effectCitation8. Animal studies have demonstrated that intravenous interferon gamma-1b produced a monophasic dose-dependent fever in rabbits and that pyrogenic tolerance was observed following three successive daily injectionsCitation11. The mechanism by which injected interferon gamma-1b evokes the acute pattern of fever is unclear. It is likely that interferons, acting directly or indirectly via other cytokines such as IL-1, resets the temperature control center, probably via PGE2 at a sub-thalamic levelCitation12,Citation13,Citation14. In the current study, fever was the individual symptom that most consistently showed sensitivity to duration of therapy and treatment regimen. This was particularly the case for the FLS profile at 8 and 12 hours post-injection. Peak interferon gamma-1b levels occur at approximately 7 hours after subcutaneous injection which is consistent with the observed FLS severity observed at 8 hours post-injection. It was noted that the earlier 4 hour FLS assessment was not significantly influenced by fever but rather seemed to be sensitive to chills and tiredness. The relationship between the different individual FLS symptoms associated with cytokine administration is unclear. While chills and myalgias have commonly been viewed as secondary to a pyrogenic effect, there is also evidence that symptoms such as chills can be dissociated from any increase in body temperatureCitation15. This raises the prospect that FLS is a complex combination of individual symptoms with distinct time courses and that a composite score methodology at multiple time points post-injection, as adopted in this study, is best suited to capturing the clinical pattern of FLS following interferon gamma-1b administration.
There was a high (37.5%) discontinuation rate in this study. The majority of discontinuations were not adverse event related but reflect withdrawal of consent by subjects based on the burden of 12–14 hour visits three times weekly for 6 weeks. Of the adverse event related discontinuations, three were during the No Titration phase and the four AEs classed as severe were also all reported during the No Titration phase. While it is not possible to extrapolate these adverse event trends to the clinical setting, it does support a potential improved safety/tolerability with the titration regimen.
There were three particular limitations to this study. The first relates to a lack of formal validation of the FLS measurement instrument and the second relates to this study being conducted in healthy subjects rather than in patients with disease of interest. The third limitation relates to the relatively high discontinuation rate. Despite these limitations, this study does support the potential utility of a titration regimen for initiation/re-initiation of interferon gamma-1b therapy.
Conclusion
In conclusion, this study demonstrated that a short 2 week, two-step dose titration regimen reduces the severity of FLS in healthy male and female subjects initiating interferon gamma-1b therapy.
Transparency
Declaration of funding
This study was supported by Vidara Therapeutics Research Ltd.
Declaration of financial/other relationships
J.G.D. and M.L.M. have disclosed that they are employees and shareholders of Vidara Therapeutics Research Ltd. M.A.M. has disclosed that he is an employee of Prism Research Inc., a contract research organization contracted to conduct this study. He has no other significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers on this manuscript received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Acknowledgments
The study was project managed and monitored by IND2Results and statistical analysis was provided by Applied Statistics and Consulting.
Previous presentation: A preliminary presentation of data was submitted in abstract form to the Clinical Immunology Society (CIS) and was accepted for poster presentation at the 2014 Annual Meeting in Baltimore, MD, 10–13 April 2014.
Notes
*Actimmune is a registered trademark of Vidara Therapeutics International Ltd.
References
- Descotes J, Vial T. Flu-like syndrome and cytokines. In: House RV, Descotes J. Methods in Pharmacology and Toxicology: Cytokines in Human Health: Immunotoxicology, Pathology and Therapeutic Applications. Totowa, NJ: Humana Press Inc. 2007
- Frohman E, Phillips T, Kokel K, et al. Disease-modifying therapy in multiple sclerosis: strategies for optimizing management. The Neurologist 2002;8:227-36
- Wroe SJ. Effects of dose titration on tolerability and efficacy of interferon beta-1b in people with multiple sclerosis. J Int Med Res 2005;33:309-18
- Brandes DW, Bigley K, Hornstein W, et al. Alleviating flu-like symptoms with dose titration and analgesics in MS patients on intramuscular interferon beta-1a therapy: a pilot study. Curr Med Res Opin 2007;23:1667-72
- Matson MA, Zimmerman TR Jr, Tuccillo D, et al. Dose titration of intramuscular interferon beta-1a reduces the severity and incidence of flu-like symptoms during treatment initiation. Curr Med Res Opin 2011;27:2271-8
- Winkelstein JA, Marino MC, Johnston RB Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155-69
- Sobacchi C, Schulz A, Coxon FR, et al. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 2013;9:522-36
- The international chronic granulomatous disease cooperative study group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Eng J Med 1991;324:509-16
- Marciano BE, Wesley R, De Carlo ES, et al. Long-term interferon-ɤ therapy for patients with chronic granulomatous disease. Clin Infect Dis 2004;39:692-9
- Dinarello CA, Bemheim HA, Duff GW, et al. Mechanisms of fever induced by recombinant human interferon. J Clin Invest 1984;74:906-13
- Morimoto A, Murakami N, Takada M, et al. Fever and acute phase response induced in rabbits by human recombinant interferon-ɤ. J Physiol 1987;391:209-18
- Siegert R, Phillip-Dormston WK, Radsak K, Menzel H. Mechanism of fever induction in rabbits. Infect Immun 1976;14:1130-7
- Netea MG, Kullberg BJ, Van der Meer JWM. Circulating cytokines as mediators of fever. Clin Infect Dis 2000;31:S178-84
- Blatteis CM, Sehic E, Li S. Pyrogen sensing and signaling: old views and new concepts. Clin Infect Dis 2000;31(Suppl 5):S168-77
- Guieu JD, Hellon RF. The chill sensation in fever. Plugers Arch 1980;384:103-4

