Abstract
Objectives:
Medication prescribing information provides guidance to healthcare providers on how to prescribe a drug properly. Oftentimes patient factors in addition to the prescribing information are considered when selecting medications. Utilizing real-world pharmacy and medical claims data, this study assessed US practitioner prescribing practices of US approved transdermal buprenorphine system (BTDS) in relation to BTDS’s full prescribing information (FPI) as well as the relationship between patient factors and initial BTDS dose.
Research design and methods:
Patients aged ≥18 years initiating BTDS between 1 January 2011 and 30 November 2011 were identified in the IMS Pharmacy and Private Practitioner Medical Claims databases. The index date was defined as the first filled BTDS prescription. Demographics, chronic pain-related medical conditions in the 12 months pre-index and prior medication use in the 6 months pre-index were assessed. Initial BTDS dosing strength, receipt of approved initial BTDS dose per the FPI, and concomitant medications were assessed in the post-index 6 month period.
Results:
The study included 10,457 patients newly treated with BTDS. The majority of patients were female (69.9%) with a mean (±SD) age of 54.5 (±15.2) years. Within the 6 months prior to the index BTDS prescription, 91.7% of the patients used opioids. Overall, 48.9% of patients were prescribed the FPI approved BTDS dose. When stratified, 73.5% of opioid-naïve patients received the FPI approved initial dose compared to 46.0% of those with prior opioid experience of ≤80 mg morphine-equivalent daily dose. Patients on BTDS alone (i.e. monotherapy) had a higher rate of receiving the FPI approved initial BTDS dose compared to patients on BTDS concomitant regimens (p < 0.05).
Conclusions:
Practitioners demonstrated that they prescribe in accordance with BTDS’s prescribing information in the majority of opioid-naïve patients and in approximately half of opioid-experienced patients. The initial opioid dose is a critical step in treatment, setting the stage for preventing side-effects and improving treatment effectiveness. Understanding practitioner prescribing practices with regard to the initial dose selection of BTDS may provide insight on how to improve outcomes of care and reduce healthcare resource utilization and costs associated with pain management.
Limitations:
Data obtained from prescription claims reflect only the activities of prescriptions filled, not medication use or other clinical characteristics observed by physicians when treating patients.
Introduction
Chronic pain is widespread throughout the United States and affects a population greater than diabetes, heart disease and cancer combinedCitation1. It has been estimated that approximately 30% of the American public or 100 million Americans suffer from chronic pain each day, of which many experience chronic non-cancer painCitation2,Citation3. As physicians evaluate an appropriate treatment for the patient, the undertreatment as well as overtreatment (e.g. too high of a dose) of chronic pain can affect many aspects of a patient’s life such as reduction in overall quality of life, inhibition to work effectively, and interference with normal daily activitiesCitation4. In addition, chronic pain has an effect on society, creating a drain on healthcare resources and increasing medical costs. A recent report has indicated that chronic pain costs approximately $560–635 billion annually in the United States, with approximately $297–$336 billion due to lost productivityCitation2.
Chronic pain is a complex condition; it is not simply a reflection of peripheral inputs or pathology but is a dynamic reflection of central neuronal plasticity. This plasticity profoundly alters sensitivity to an extent that it is a major contributor to many clinical pain syndromesCitation5. Maladaptive pain states, including inflammatory/joint pain, neuropathic pain, and non-inflammatory/non-neuropathic pain often result from peripheral and central sensitizationCitation6. As a result, achieving optimal pain management in patients suffering from chronic moderate-to-severe pain is a challenge. Barriers to optimal pain management comprise both patient and healthcare provider centric issues as well as insurance coverage and the availability of treatment options. Individual barriers of healthcare professionals may include: the lack of knowledge of current treatment options; inadequate assessment skills; regulatory scrutiny/concerns over prescribing controlled substances; assessment bias which can be in the form of underassessment or a disparity between clinician’s and patient’s ratings of pain severity; healthcare provider time constraints and poor clinician–patient communicationCitation7,Citation8. Restrictions set forth by insurance carriers and/or pharmacies may interfere with physician prescribing decisions and may not necessarily lead to better outcomes.
Whatever the type of pain therapy selected, it should be tailored to fit individual patient needs. Options to treat chronic moderate-to-severe pain allow for individualization of therapy, which is essential because pain is personalized. This personalization leads to different choices being made for the patient whose job consists of physical labor; the patient who is elderly and/or taking numerous medications for other medical conditions; and the patient with a history of alcoholism or drug abuse. The choice of therapy for one pain condition may not be the right choice for other pain conditions and the right choice for one patient is not always right for every patient. Pain management utilizing opioids has this challenge, leaving prescribers to tailor treatment to the needs of individual patients thus avoiding the ‘one size fits all’ approach.
Optimal use of opioids is complex and to achieve effective analgesia one generally must identify the right opioid, right dosing interval and right route of administration. Guidelines and medication prescribing information have been developed to aid in prescribing practices and achieve opioid therapeutic effectiveness, but data regarding their use is limited, especially for newer marketed productsCitation9–17. A 7 day buprenorphine transdermal system (BTDS, Butrans) has been approved in the US for management of moderate-to-severe chronic pain in patients requiring a continuous, around-the-clock opioid analgesic for an extended period of timeCitation18. BTDS is available as a 7 day transdermal patch in three different strengths (5 mcg/hr, 10 mcg/hr, 20 mcg/hr). Transdermal buprenorphine has been shown to be effective and safe in chronic pain patients with low back pain and osteoarthritis as well as in patients with neuropathic painCitation19–27. The BTDS’s prescribing regimen outlined in the full prescribing information (FPI) lists the approved initial dose based on prior opioid experienceCitation18. Due to its relatively recent launch (July 2010), little is known regarding US practitioner prescribing practices of BTDS and whether practitioners are following the FPI, as well as considering other factors such as concomitant medications or co-morbidities when initiating BTDS, which may lead to variance in prescribing practices.
Previous work has identified factors that are associated with BTDS persistence and dose modification in the USCitation28. In this study, the aim was to assess the consistency between physicians’ prescribing practices and the prescribing information outlined initial BTDS dose as well as identify patient characteristics and factors observed in relation to initial BTDS dosing. In addition, these factors may provide a valuable understanding of patients with chronic pain and is an important foundation for designing future comparative and health outcomes studies of pain relief.
Methods
Database
This retrospective study utilized Health Insurance Portability and Accountability Act 1996 (HIPAA) compliant, encrypted and de-identified patient level claims provided by IMS Health. The database is composed of private practitioner claims (Centers for Medicare and Medicaid Services – 1500 form) and pharmacy claims (National Council for Prescription Drug Programs, NCPDP V5.1). The private practitioner claims represent approximately one third of all private practice visits in the US. The prescription claims represent dispensed prescriptions from approximately 50–60% of all US retail pharmacies.
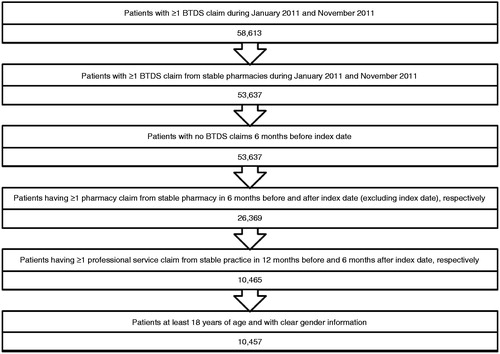
Patient selection
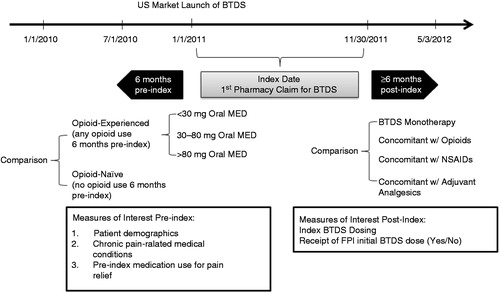
The database was queried for US patients with pharmacy claims for BTDS between 1 January 2011 and 30 November 2011 (). The index date was established as the first filled pharmacy claim for BTDS during the defined study period, plus a 6-month retrospective, ‘look-back’ period to 1 July 2010. Inclusion criteria required patients to (1) have at least one pharmacy claim for BTDS submitted from a stable pharmacy between January 2011 and November 2011; (2) have at least one pharmacy claim for any use during the 6 months of the pre- and post-index period, respectively; (3) have at least one practitioner service claim for any reason during the 12 months of the pre-index period and the 6 months of post-index period, respectively; (4) be at least 18 years of age as of the index date; (5) have known information of gender.
Patients were further classified into cohorts based on (1) prior opioid experience and (2) concomitant medication use in the 30 days post-index date. Patients were considered opioid-experienced if a pharmacy claim for an opioid occurred during the pre-index period (≤6 months), otherwise they were classified as opioid-naive. Opioid cohorts were created based on the FPI’s listed cohorts for initial BTDS dose which consists of patients taking <30 mg morphine equivalent daily dose (MED), 30–80 mg MED and >80 mg MED. MED was calculated based on last opioid prescription prior to BTDS index date. The conversion factors were based on prior published literatureCitation12,Citation29,Citation30.
To categorize patients based on post-index concomitant medication use, the following hierarchical approach was used: patients were classified as opioid concomitant if patients received BTDS and another opioid within 30 days post-index date. If patients did not have a claim for an additional opioid, patients were then checked for a prescription claim for a nonsteroidal anti-inflammatory drug (NSAID) within 30 days post-index. If patients did not have a claim for a NSAID, patients were then checked for a prescription claim for an adjuvant medication within 30 days post-index. Adjuvant analgesics were defined as tricyclic antidepressants, anticonvulsants, duloxetine, milnacipran, central muscle relaxants and local anesthetics (capsaicin and lidoderm). If patients did not have any of these types of prescription claims within 30 days post-index, they were classified into the BTDS monotherapy cohort.
Study outcome measurements
Patient demographics, chronic pain-related medical conditions and pain-related medication use were identified during the pre-index period (≤6 months). In the post-index (≥6 month) period, concomitant medications, index BTDS dosing strength and receipt of BTDS dose were evaluated ().
Statistical analysis
Statistical analyses were primarily descriptive in nature. Categorical measures were reported for patient counts (N) and percentages. Continuous variables were presented as the mean, standard deviation and median. Appropriate statistical tests including chi-squared test for categorical variables and Student's t-test or ANOVA test for continuous variables were used to evaluate differences in outcomes between comparator cohorts. An a priori alpha threshold of 0.05 was considered statistically significant.
Results
Patient baseline characteristics
A total of 58,613 US patients were identified to have at least one pharmacy claim for BTDS during January 2011 to November 2011. After applying the inclusion and exclusion criteria a total of 10,457 patients met the qualifying study criteria and were analyzed (). The majority of patients were female (69.9%); 75.4% of the patients were under the age of 65 years, with a mean (±SD) age of 54.5 years (±15.2). Patients were located geographically in the South (42.7%), the largest US census region, followed by the West (20.3%), Midwest (18.3%) and the Northeast (16.5%). Commercial insurance, Medicare Part D, Medicaid and other/unspecified carrier accounted for 29.4%, 10.7%, 3.9%, and 55.9% of payer types, respectively.
Patient characteristics prior to BTDS initiation
Prior to BTDS initiation, patient medications primarily consisted of opioids (91.7%), adjuvant analgesics (59.0%), NSAIDs (34.7%), proton pump inhibitors (27.9%) and oral corticosteroids (26.6%). Patients on prior opioid treatment were generally prescribed a morphine equivalent daily dose (MED) of 30–80 mg (53.4%) (). Patients’ co-morbid conditions 12 months prior to initiating BTDS were assessed. For the entire patient population, the majority of patients had back pain (68.8%), followed by musculoskeletal pain (58.5%), osteoarthritis (40.6%), trauma (34.1%), post-surgery care (27.3%), fibromyalgia (19.8%), limb pain (18.0%), neuropathic pain (16.9%), chronic pain (16.4%), and cancer (11.9%). In addition, opioid-experienced patients had significantly higher rates of nearly all identified pain conditions (except cancer) compared to opioid-naïve patients (p < 0.05).
Table 1. Patient characteristics pre and post BTDS index date.
Receipt of full prescribing information approved BTDS dose
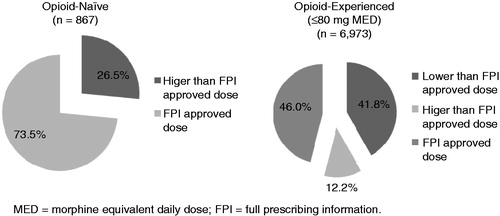
In order to understand the consistency between prescriber practices and the FPI indicated BTDS dose, patients’ MEDs were calculated and used to group them based on the FPI listed MED groups (e.g. <30 mg, 30–80 mg, >80 mg). A total of 7840 patients who were opioid-naïve or received prior MED dose <80 mg were analyzed in order to determine the percentage of patients who were prescribed the FPI approved BTDS dose by their physician. Patients who had a recorded prior MED dose >80 mg were excluded from the analysis since the FPI does not identify a BTDS dose for this patient populationCitation18. Approximately half (49.0%) of patients received the FPI approved initial BTDS dose (). A majority of the opioid-naïve patients (73.5%) received the approved initial dose versus 46.0% of the opioid-experienced patients (p < 0.05) (). Among opioid-experienced users (n = 6,973), 65.4% of those previously on MED dose <30 mg received the FPI approved dose of BTDS. This was significantly higher (p < 0.05) than those previously on MED of 30–80 mg (41.1%).
Table 2. Percentage of patients prescribed the FPI approved BTDS dose.
Factors related to initial BTDS dose
In order to gain a better understanding of why the dispensed strength varied from the FPI approved dose, we analyzed a variety of patient characteristics that may have influenced the prescribed dose. Analysis of the total population of patients initiated on BTDS revealed that 52.3%, 40.0%, and 7.7% of patients received 5 mcg/hr, 10 mcg/hr, or 20 mcg/hr as initial dose, respectively (). To determine whether prior opioid experience had an effect on initial dosing regimen, patients were grouped based on prior opioid experience and the initiated BTDS dose; 73.5% of patients classified as opioid-naïve were initiated on the 5 mcg/hr patch compared to 50.3% of patients who were considered opioid-experienced (p < 0.05). Higher initial doses were generally given to those who were more opioid-experienced (e.g. higher MED). For example, stratified by prior MED <30 mg, 30–80 mg, and >80 mg, analyses indicated that 65.4%, 52.3% and 38.3% of patients, respectively, were started on the 5 mcg/hr dose (p < 0.05).
To determine whether concomitant medication during BTDS initiation was a factor associated with the starting dose, medication post-index pharmacotherapy (1 to 30 days) was assessed (). Using a hierarchical classification, 21.8% of patients started BTDS as a monotherapy while 64.2%, 4.6%, and 9.4% of patients used additional pain-relief medications such as additional opioids, NSAIDs, or adjuvant analgesics, respectively. The top six opioids used concomitantly with BTDS included: hydrocodone (39.8%), oxycodone (30.0%), tramadol (9.6%), fentanyl (4.1%), morphine (3.6%), and tapentadol (3.4%).
Slightly more than half of the patients (50.7%) who initiated BTDS with other concomitant medications initiated BTDS at the higher dosage strengths (10 mcg/hr or 20 mcg/hr). Within the concomitant sub-groups, analyses indicated that 53.5%, 35.4%, and 40.0% of patients taking BTDS with either an additional opioid or NSAID or adjuvant analgesics, respectively, started on a dosage strength >5 mcg/hr, compared to 36.9% of patients on monotherapy (p < 0.05). Additionally, patients on monotherapy had a higher rate of receiving the approved initial BTDS dosage strength compared to patients on BTDS and other concomitant regimens (p < 0.05) ().
Discussion
In this retrospective cohort analysis, the consistency between US practitioner prescribing and BTDS’s FPI initial dose was evaluated. In our study, approximately half of the patients received an initial BTDS prescription consistent with the dose initiation paradigm described in the FPI. Practitioners generally prescribed the FPI dose to opioid-naïve patients (73.5%) and less often to opioid-experienced patients (46.0%). In addition, 37.2% of opioid-experienced (≤80 mg MED) patients were prescribed a dose lower than the FPI outlined dose and 13.8% of both opioid-experienced and opioid-naïve patients were prescribed a higher dose. This data indicates that there are factors in addition to prior opioid experience that practitioners consider when initiating BTDS.
Our study identified concomitant medications as a factor that practitioners may consider when initiating BTDS. In addition, practitioners may be considering other patient characteristics that are not generally captured in pharmacy claims database. BTDS is a relatively recent addition to the armamentarium of analgesics available to practitioners to relieve moderate-to-severe pain and information regarding its use within the United States is limited. Practitioners may therefore rely more on their prescribing experiencesCitation31 and on the current opioid guidelines when deciding on BTDS initiation dose. Guidelines stress the importance of a comprehensive initial evaluation which entails assessing the pain (e.g. cause of pain, severity) and prior treatment of pain (e.g. appropriateness, adequacy, and outcomes) as well as the effects of pain on the person’s life and function (e.g. activities of daily living, functional status). For example, patients with differences in pain severity such as moderate (4–6 on numerical rating scale [NRS] of 0–10) versus severe (7–9 on NRS of 0–10) or pain type such as chronic low back pain versus osteoarthritis may be prescribed differently. In addition, it is suggested that social factors and medical/mental health conditions are also reviewed. This may entail an evaluation of substance abuse history, identifying psychiatric conditions that may affect treatment compliance and adherence, social history/status (e.g. employment, marital status, social network), and presence of medical conditionsCitation12,Citation17,Citation32. A study assessing the factors associated with higher doses of opioids in a primary care setting for lower back pain identified higher co-morbidity as an influential factorCitation33. In a knowledge, attitudes, and practice study regarding management of moderate-to-severe chronic pain with opioids, physicians took into consideration sleeping, walking, maintaining an independent lifestyle, and job responsibilities when managing patientsCitation34.
Our study identified a patient population (37.2%) that received a lower dose than outlined by the BTDS FPI. One reason for this may be that physicians are cautious about adverse events during the initiation and titration period, which may result in premature discontinuation. Another reason may be that they are attempting to adhere to the well established and well promoted opioid guidelines. Guidelines often suggest opioids be initiated at a low dose in order to minimize the development of side-effects, this relates to the common concept of ‘start low and go slow’. For example, guidelines set forth by the American Pain Society recommend opioids be started at a low dose and titrated slowly if patients are opioid-naïve; have minimal opioid exposure or present with co-morbidities such as obesity, frailty or poor general health, liver or renal disease; or are on other medicationsCitation17. Guidelines set forth by the VA recommend starting at a lower dose in order to gauge initial response, minimize adverse effects, and allow patients to develop tolerance prior to making dose adjustmentsCitation12. Additionally, physicians may be trying to switch patients from their current opioid regimen by rotating to BTDS and utilizing its convenient route of administration and dosing schedule. Further study is warranted to understand why practitioners may initiate a lower BTDS dose than the FPI approved dose.
The BTDS FPI recommends consideration of a patient’s prior opioid experience as a factor to consider in calculating the starting dose. Possible outcomes associated with suboptimal dosing may include inadequate pain control, increased risk of experiencing adverse effects, poor medication persistence and an impact on patients’ quality of life. Patients receiving lower doses than required may fail to experience a reduction in pain control, while those receiving higher doses than required may develop side-effects. Variations in dosing may decrease medication adherence, compliance and persistence, which has shown to impact healthcare costs and resource utilizationCitation35 as a result of increased number of visits to doctor’s office, increased prescription writing to mitigate side-effects, etc. All of these outcomes will inevitably affect a patient’s overall quality of life, which includes physical wellbeing, general health, vitality, psychological or mental wellbeing and social functioning. Regardless of reason for prescribing a higher or lower dose than the FPI approved dose, it is important to monitor these patients for both tolerance and analgesic response to avoid inadequate treatment of their pain.
Clinical perspective
The authors suggest that prescribers do not deviate from the prescribing instructions in the package insert. Since BTDS is relatively new to the market, it is felt that many prescribers might not be fully versed with the package insert and therefore should devote the necessary time to read and understand the full prescribing information. Initial dose selection should follow the prescribing instruction and be guided both by the prescriber’s clinical judgment and published chronic opioid therapy (COT) guidelinesCitation17.
Study limitations
Several limitations should be considered in interpreting the results of this retrospective claims analysis. The data is specific to the US approved buprenorphine transdermal patch and the prescribing practices in the US; data may not be valid for other countries where rates of opioid use may be different and a higher strength (35 mcg/hr, 52.5 mcg/hr, 70 mcg/hr) buprenorphine transdermal patch is approvedCitation36,Citation37. Prescription claims data do not explicitly state the type of pain BTDS was prescribed for, reasons for dosage selection or why a dose other than that indicated in the FPI was prescribed. In addition, the study was not able to determine whether patients properly used the medication as prescribed. Pharmacy claims data reflect only the filling of prescriptions but not whether the patient takes the medications as prescribed. This limitation may have impacted the interpretation of ‘concomitant’ use of drugs as defined in this study. What was defined as ‘concomitant’ use could reflect actual co-treatment with BTDS and the other drug, or may reflect a switch from BTDS to the second drug. In addition, a breakdown by the type of opioid or by product name was not performed for those with prior opioid experience and this may have been a factor in rotation/switching to BTDS.
Conclusion
In a real world setting, approximately half of the patients receiving an initial BTDS prescription are prescribed an initial dose consistent with the FPI. When initiating BTDS, practitioners may consider concomitant medications along with prior opioid experience. Based on the evidence underlying the FPI, safety and efficacy are likely enhanced when the FPI guidelines are followed. Future studies should explore how initial dosing affects medication adherence, compliance, and persistence as well as efficacy, safety, costs, and health outcomes.
Transparency
Declaration of funding
Funding of the study was provided by Purdue Pharma, Stamford, CT, USA.
Declaration of financial/other relationships
J.V.P. has disclosed that he is a consultant for Johnson & Johnson, Purdue Pharma LP, Baxter International Inc., Endo Pharmaceuticals Inc., Insys Therapeutics and Collegium Pharmaceutical. R.B.-J. has disclosed that he is an employee of Purdue Pharma. G.H. has disclosed that he was employed by IMS Health at the time of the study, which received study funding from Purdue Pharma LP. He is now an employee of Symphony Health Solutions. C.-L.C. has disclosed that she is an employee of IMS Health.
CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgments
The authors thank Dhvani Shah MS for her assistance during data analysis and manuscript preparation.
Notes
*Butrans is a registered trade name of Purdue Pharma, Stamford, CT, USA
References
- The American Academy of Pain Medicine. AAPM Facts and Figures on Pain. 2013. Available at: http://www.painmed.org/patientcenter/facts_on_pain.aspx [Last accessed 11 April 2013]
- Institute of Medicine Report from the Committee on Advancing Pain Research. Relieving Pain in America, A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: The National Academies Press, 2011
- Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 2010;11:1230-9
- American Pain Foundation. Voices of Chronic Pain: A National Study Conducted for American Pain Foundation. New York, NY: David Michaelson & Company; 2006
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895-926
- Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004;140:441-51
- Berry PH, Chapman CR, Covington EC, et al. Pain: Current Understanding of Assessment, Management, and Treatments. Reston, VA: National Pharmaceutical Council Inc. and the Joint Commission on Accreditation of Healthcare Organizations, 2001
- Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. The J Am Board Fam Pract 2001;14:211-18
- The British Pain Society. Opioids for Persistent Pain: Good Practice. London, WC: The British Pain Society, 2010
- American Pain Society in Conjunction with The American Academy of Pain Medicine. Guideline for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain: Evidence Review. Chicago, IL: American Pain Society; 2009
- Kahan M, Mailis-Gagnon A, et al. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 1: general population. Can Fam Physician 2011;57:1257-66, e407-18
- Management of Opioid Therapy for Chronic Pain Working Group. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. Washington, DC: Department of Veterans Affairs, Department of Defense; 2010
- Washington State Agency Medical Directors' Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid treatment. Olympia, WA: Washington State Department of Labor and Industries; 2010
- Ambrosio F, Finco G, Mattia C, et al. SIAARTI recommendations for chronic noncancer pain. Minerva Anestesiologica 2006;72:859-80
- Coluzzi F, Pappagallo M. Opioid therapy for chronic noncancer pain: practice guidelines for initiation and maintenance of therapy. Minerva Anestesiologica 2005;71:425-33
- Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) Guidelines for Responsible Opioid Prescribing in Chronic Non-Cancer Pain: Part 2 – Guidance. Pain Physician 2012;15(3 Suppl):S67-116
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10:113-30
- Purdue Pharma. BTDS (buprenorphine) Transdermal System – Full Prescribing Information. 2012
- Breivik H, Ljosaa TM, Stengaard-Pedersen K, et al. A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis patients naïve to potent opioids. Scand J Pain 2010;1:122-41
- Gordon A, Callaghan D, Spink D, et al. Buprenorphine transdermal system in adults with chronic low back pain: a randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clin Therapeut 2010;32:844-60
- Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag 2010;15:169-78
- James IGV, O'Brien CM, McDonald CJ. A randomized, double-blind, double-dummy comparison of the efficacy and tolerability of low-dose transdermal buprenorphine (BuTrans seven-day patches) with buprenorphine sublingual tablets (Temgesic) in patients with osteoarthritis pain. J Pain Symptom Manag 2010;40:266-78
- Landau CJ, Carr WD, Razzetti AJ, et al. Buprenorphine transdermal delivery system in adults with persistent noncancer-related pain syndromes who require opioid therapy: a multicenter, 5-week run-in and randomized, double-blind maintenance-of-analgesia study. Clin Therapeut 2007;29:2179-93
- Karlsson M, Berggren A-C. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 microg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: a 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin Therapeut 2009;31:503-13
- Guetti C, Angeletti C, Marinangeli F, et al. Transdermal buprenorphine for central neuropathic pain: clinical reports. Pain Practice 2011;11:446-52
- Steiner D, Munera C, Hale M, et al. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J Pain 2011;12:1163-73
- Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naive patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manag 2011;42:903-17
- Pergolizzi JV, Ben-Joseph R, Chang CL, Hess G. Predicting medication persistence to buprenorphine transdermal system. Pain Practice 2014 [Epub ahead of print]
- Cass LJ. Pentazocine vs morphine. JAMA 1964;189:332
- Janssen Pharmaceuticals. Duragesic (Fentanyl Transdermal System) – Full Prescribing Information. 2012
- Smith HS. Opioid Therapy in the 21st Century. New York, NY: Oxford University Press, Inc., 2008
- Utah Department of Health. Utah Clinical Guidelines on Prescribing Opioids for Treatment of Pain. Salt Lake City, Utah: Utah Department of Health, 2009
- Kobus AM, Smith DHM, Morasco BJ, et al. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain 2012;13:1131-8
- Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin 2010;26:1579-85
- New England Healthcare Institute. Thinking outside the pillbox: a system-wide approach to improving patient medication adherence for chronic disease. Available from: http://www.nehi.net/publications/17-thinking-outside-the-pillbox-a-system-wide-approach-to-improving-patient-medication-adherence-for-chronic-disease/view. [Last accessed 22 March 2014]
- Budd K. Buprenorphine and the transdermal system: the ideal match in pain management. Int J Clin Pract 2003;133:9-14; discussion 23-4
- Bohme K. Buprenorphine in a transdermal therapeutic system – a new option. Clin Rheumatol 2002;21(Suppl 1):S13-16