Abstract
Objective:
Systematically review and synthesize the clinical evidence of treatments for attention deficit hyperactivity disorder (ADHD) by indirectly comparing established treatments in the UK with a drug recently approved in Europe (lisdexamfetamine [LDX]).
Research design and methods:
Population: children and adolescents. Setting: Europe. Comparators: methylphenidate (MPH), atomoxetine (ATX), and dexamphetamine (DEX). Electronic databases and relevant conference proceedings were searched for randomized, controlled clinical trials evaluating efficacy and safety of at least one of the comparators and LDX. Quality assessments for each included trial were performed using criteria recommended by the Centre for Reviews and Dissemination. Network meta-analysis methods for dichotomous outcomes were employed to evaluate treatment efficacy.
Main outcome measures:
Response, as defined by either a reduction from baseline of at least 25% in the ADHD Rating Scale [ADHD-RS] total score or, separately, as assessed on the Clinical Global Impression–Improvement [CGI-I] scale, and safety (all-cause withdrawals and withdrawal due to adverse events).
Results:
The systematic review found 32 trials for the meta-analysis, including data on LDX, ATX, and different formulations of MPH. No trials for DEX meeting the inclusion criteria were found. Sufficient data were identified for each outcome: ADHD-RS, 16 trials; CGI-I, 20 trials; all-cause withdrawals, 28 trials; and withdrawals due to adverse events, 27 trials. The relative probability of treatment response for CGI-I (95% confidence intervals [CI]) for ATX versus LDX was 0.65 (0.53–0.78); for long-acting MPH versus LDX, 0.82 (0.69–0.97); for intermediate release MPH versus LDX, 0.51 (0.40–0.65); and for short-acting MPH versus LDX, 0.62 (0.51–0.76). The relative probabilities of ADHD-RS treatment response also favored LDX.
Conclusions:
For the treatment of ADHD, the synthesis of efficacy data showed statistically significant better probabilities of response with LDX than for formulations of MPH or ATX. The analysis of safety data proved inconclusive due to low event rates. These results may be limited by the studies included, which only investigated the short-term efficacy of medications in patients without comorbid disorders.
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common neurobehavioral disorders in childhoodCitation1. In a systematic review of 102 studies, the prevalence of ADHD was estimated to be 5.29% in children worldwide, 5% in European children, and 6.0% to 6.5% in children in North AmericaCitation2. According to a national survey in the United States in 2007, parent-reported prevalence of ADHD in children aged 4 to 17 years was 9.5%, representing 5.4 million children. Further, boys were more likely than girls to have ever been diagnosed with ADHD (13.2% vs. 5.6%, respectively)Citation3. Although ADHD does persist into adulthood in up to 50% of patients, the focus of this systematic review is on ADHD in children and adolescentsCitation4.
The core symptoms of ADHD include chronic levels of inattention, impulsiveness, and hyperactivityCitation1. Children with ADHD often fail to achieve their potential and many have comorbid difficulties such as delayed development, specific learning problems, and other emotional and behavioral disordersCitation5. ADHD often results in excessively demanding or attention-seeking behavior, noisy or disruptive behavior, aggressive or defiant behavior, and impulsive or risk-taking behaviorCitation6. ADHD is associated with an increased risk of accidents, cigarette smoking, and substance abuseCitation7–11. Consequently, across different measures, ADHD leads to impaired health-related quality of life, estimated at 1.5–2 standard deviations below the appropriate population norms on different parent-rated scalesCitation12.
The provision of treatments and interventions for children and adolescents who have ADHD is varied but often includes psychological therapy, parent training, and medicationsCitation5,Citation13. In the United Kingdom, methylphenidate (MPH), atomoxetine (ATX), and dexamphetamine (DEX) are licensed for the treatment of ADHD in children and adolescents. In technology appraisal TA98, the National Institute for Health and Clinical Excellence (NICE) concluded that these medications are effective in controlling the symptoms of ADHD relative to no treatment. NICE recommends that MPH is considered as first-line treatment for ADHD without significant comorbidity or for ADHD with comorbid conduct disorder. ATX is typically reserved for patients who have tried MPH; however, ATX can be used as a first-line treatment when tics, Tourette’s syndrome, anxiety disorder, stimulant misuse, or risk of stimulant diversion is presentCitation13. Other medications, including atypical antipsychotics, bupropion, nicotine, clonidine, modafinil, tricyclic antidepressants, and other antidepressants are not licensed for treatment of ADHD, but are occasionally prescribed to patients who do not respond to licensed medications. These drugs were not included in TA98Citation14. The use of ATX, DEX, and MPH has also been evaluated and recommended Europe-wideCitation15.
Lisdexamfetamine (LDX; Elvanse) is a recently approved pharmacological treatment option for ADHD in Europe. LDX is indicated as part of a comprehensive treatment program for ADHD in children aged 6 years and older whose response to previous methylphenidate treatment is considered clinically inadequateCitation16. The short-term and long-term (up to 1 year) safety and efficacy in children and adolescents has been demonstrated in several trials in the US and EuropeCitation16.
Given the recent availability of trial results for LDXCitation17–19, it is appropriate to assess the efficacy of LDX relative to other common ADHD treatments. A previous meta-analysis suggests that LDX potentially demonstrates better efficacy than MPH by contrasting effect sizes for total ADHD symptoms, hyperactivity–impulsivity, and inattentionCitation20. However, that published meta-analysis made no formal statistical comparisons between active treatmentsCitation20. This meta-analysis is the first comparative assessment of LDX against other licensed medications and makes use of all relevant evidence defined by the UK-specific treatment recommendationsCitation13. The available evidence consists of placebo- and active-controlled trials and three-arm trials; therefore, network meta-analyses (NMA) methods were used to robustly and simultaneously combine direct and indirect treatment estimates across studies. The evidence synthesis using the NMA method that we will report in this manuscript is the first in ADHD.
Patients and methods
Search process
A systematic literature review was performed, according to a prespecified protocol, to comply with the requirements of NICE’s single technology assessmentCitation21. The following electronic databases were searched: (1) The Cochrane Library including the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and the Database of Abstracts of Reviews of Effectiveness; (2) MEDLINE and MEDLINE In-Process; and (3) Embase. In addition, electronic and manual searches of the following websites of annual conference proceedings were conducted: American Psychiatric Association (www.psych.org), American Academy of Child and Adolescent Psychiatry (www.aacap.org), International Congress on ADHD (published in the journal ADHD Attention Deficit and Hyperactivity Disorders and indexed in Medline). Finally, bibliographic reference lists of any identified systematic reviews and meta-analyses were searched for any additional relevant clinical studies. The search targeted studies published between 1960 and April 15, 2011; any relevant unpublished at that time LDX trials were also included. The search applied no limitations on publication language.
Search terms included combinations of free text and medical subject headings. Full search strategies are presented in the online supplement. The search strategy for each database is available from the authors upon request. The search relied on four sets of terms: health condition of interest (ADHD and hyperkinetic disorder and derivatives thereof), population (children and adolescents [13–17 years old]), study type (randomized, controlled clinical trials), and intervention (drug therapy). Appropriate terms were combined and iterative searches using other relevant terms and concepts were performed.
Inclusion criteria
In addition to the inclusion criteria defined by the search terms, trials eligible for inclusion in the meta-analysis had to include at least one of the following active interventions (data for MPH were analyzed separately based on its three different lengths in duration of action):
LDX: Elvanse, Shire, Wayne, PA, USA
MPH long acting (MPH-LA) (duration of action ≥10 hours): Concerta XL, McNeil Pediatrics, Division of Ortho-McNeil-Janssen Pharmaceuticals Inc., Titusville, NJ, USA
MPH intermediate release (MPH-intR) (duration of action of 6–9 hours): Equasym XL, Shire Pharmaceuticals Ireland Limited, Dublin, Ireland; Medikinet XL, Flynn Pharma Ltd, Dublin, Ireland
MPH short acting (MPH-SA) (duration of action of 4–5 hours): e.g. Ritalin, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
ATX: Strattera, Lilly USA, LLC, Indianapolis, IN, USA
DEX: generic
Crossover trials were excluded from the meta-analysis to avoid problems with period effects in crossover trials. For example, baseline disease severity at the start of period 2 may be different when compared with period 1. Including only period 1 data from crossover trials was considered as an alternative to exclusion, but this information was not readily available in the reviewed and/or included publications. Full screening inclusion and exclusion criteria are presented in the online supplement.
Identified studies were screened and selected by two experienced literature reviewers working independently before comparing results. Any disagreements were discussed with the research team to reach a consensus about study inclusion. Data extraction was performed by an experienced literature reviewer and quality checked by the statistician responsible for the meta-analyses.
Outcomes
In clinical trials of treatments for ADHD, response to treatment is often captured by dichotomizing outcomes from two commonly used instruments: the ADHD-RSCitation22,Citation23, a parent- or teacher-completed or clinician-administered instrument used to diagnose ADHD and assess treatment response, and the Clinical Global Impressions–Improvement (CGI-I), a clinician-rated scale that assesses change over time, medication efficacy, and patient functioningCitation24. In this evidence synthesis, derived dichotomous response outcomes, separately for ADHD-RS and CGI-I, were used to evaluate relative efficacy of active treatments. Relative safety of treatments was assessed by comparing rates of all-cause and adverse-event discontinuations.
ADHD-RS response was defined as the proportion of responders with at least a 25% reduction in score (other percentage reductions were included in the absence of a 25% response). CGI-I response was defined as the proportion of responders with scores of 1 (‘very much improved’) and 2 (‘much improved’) on the CGI-I scale or on the CGI-ADHD-S (Clinical Global Impressions–ADHD–Severity) scale if a CGI-I score was not available.
Data were also extracted for the ADHD-RS (mean change from baseline and absolute score) and Conners’ Parent Rating Scales (mean change from baseline and responder rates); however, the small data quantity (reported in less than half of the included studies) did not allow for meta-analysis of these endpoints.
Statistical analyses
A mixed log-binomial model was separately fit for each outcome to estimate relative risks (RRs) and confidence intervals. The model included a fixed treatment effect; a fixed study effect; a random effect for the interaction between treatment and study; and, where appropriate, a fixed effect for a covariate term. A generic version of the NMA model can be written as follows:
where yij is the number of patients with the response, pij is the probability of response, and nij is the total number of patients in study i and on treatment j. As implemented here, the model made the following assumptions:
tj represented the logarithm of overall event probability for treatment j when xij = 0.
si represented fixed study effect. This study effect was considered to be the within-study baseline treatment effect, thus eliminating the effect of trial from the treatment comparison estimates.
vij represented random effects following a normal distribution (mean zero and unknown variance) that allowed the between-trial variability in the treatment comparison estimates to be accounted for in the overall estimates of relative treatment effects and their standard errorsCitation25.
xij represented a covariate (or covariate matrix if more than one) common to all patients in study i and on treatment j (e.g., mean age within the treatment group).
The models were fit adopting a frequentist estimation approach using PROC GLIMMIX in SAS version 9.2 (SAS Institute, Cary, NC, USA) and the methodology outlined by Jones and colleaguesCitation26. To ensure that the random effects for the treatment by study interaction correctly accounted for the between-trial treatment differences, we chose the model that constrained the random effects estimates to sum to zero within trial, by imposing symmetric correlation between the treatment effects within a trial.
Where reported separately by eligible daily doses, the analyses pooled data into combined treatment-specific estimates. NMA models were fitted for each of the four outcomes separately, and relative treatment effects for LDX were estimated versus each of the other four active treatments.
Covariates
Sex and mean patient age were individually explored for potential covariate significance, and mean baseline ADHD-RS total score was investigated as a covariate for the ADHD-RS outcome. Covariates were included in the NMA models using summary measures at the study/treatment arm level.
Sensitivity analyses
The following sensitivity analyses were performed: (1) subgroup based on ADHD-RS response, where response was strictly defined as a 25% or greater reduction from baseline score (i.e., studies using response dichotomies based on percentage reductions of 20%, 30% and 40% were excluded); and (2) a subgroup based on CGI-I response, where response was strictly defined using only responses to CGI-I (i.e., responses to CGI-ADHD-S were not included).
Heterogeneity
The results reported here include the following approaches to assess and account for heterogeneity: NMA model fit; comparison of results from adjusted indirect comparisons with head-to-head trial results; heterogeneity test for direct meta-analyses of placebo-controlled trials, sensitivity of endpoint definitions; covariate adjustment in the NMA models; and trial characteristics.
Results
Literature search results
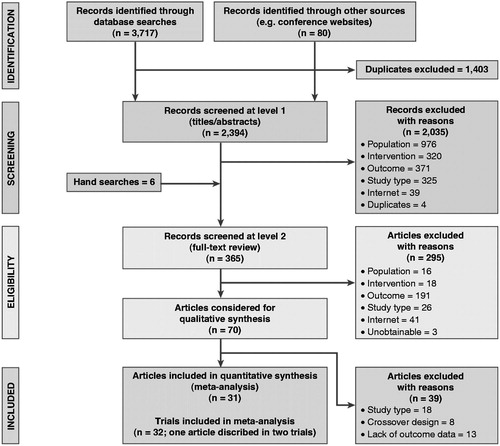
A total of 3717 titles were retrieved through database searches and a further 80 through other sources such as conference websites. After removal of duplicates, we screened 2394 titles and abstracts. After applying the screening inclusion and exclusion criteria to both the titles/abstracts and the full-text articles, 32 trials from 31 publications were eligible for meta-analysis (). One article reported two trials in a single publicationCitation27.
Quality assessment overview
Data extractors conducted quality assessments for each included trial, using quality criteria recommended by the Centre for Reviews and DisseminationCitation28 (detailed quality assessment is presented in the online supplement). Across the 32 included trials, quality reviewers generally found appropriate descriptions of randomization methods and presence of blinding. However, for 65% of the studies, the method of allocation concealment was not reported. The treatment groups within each of the studies had similar baseline characteristics, and any studies reporting imbalances in dropouts across treatment groups clearly explained the reasons for the imbalances. Relatively few of the included studies (approximately 19%) reported intent-to-treat analyses.
Evidence base
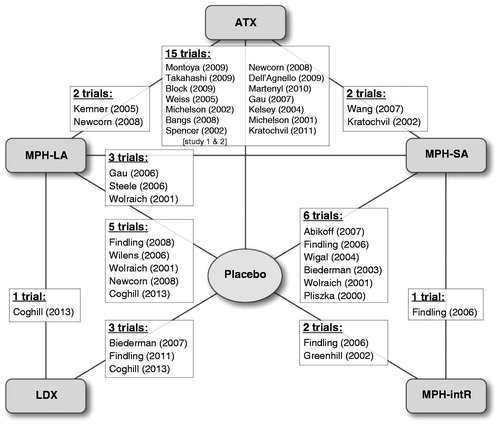
The network in shows the links between treatments and their associated trials. The networks supporting endpoint-specific analyses were typically smaller, depending on which trials contributed data to each given endpoint’s meta-analysis (not shown). Of the 32 included trials, 20 trials contributed to analyses for the CGI-I endpoint, of which 15 used the CGI-I definition and 5 used the CGI-ADHD-S definition; 16 trials contributed to analyses for the ADHD-RS endpoint, of which 12 used the ADHD-RS 25% reduction definition, 2 used a 40% reduction, 1 used a 20% reduction, and 1 used a 30% reduction; 28 trials contributed to analyses for the all-cause withdrawal endpoint; and 27 trials contributed to analyses for the adverse event withdrawal endpoint. We found only one trial reporting DEX as part of the literature searches, but this was excluded due to the crossover study designCitation17–19,Citation27,Citation29–55.
Figure 2. Network diagram of all trials included in the meta-analyses. ATX = atomoxetine; LDX = lisdexamfetamine; MPH-LA = methylphenidate long acting; MPH-intR = methylphenidate intermediate release; MPH-SA = methylphenidate short acting. SourcesCitation17–19,Citation27,Citation29–55.
Trial characteristics
Of the 32 trials, 8 adopted a forced (fixed) dosing method, 24 used titrated (optimized) dosing, and 27 included a placebo arm. There were 28 trials that included two treatment arms in the meta-analyses and 4 trials included three treatment arms. Additional details about trials, drug doses, patient characteristics, and outcome data (including imputations where appropriate) is available through the online supplement.
Baseline characteristics
gives a summary of baseline trial and patient characteristics for the five active treatments and the placebo control. Patients treated with LDX were on average older (mean age, 11.7 years) than patients treated with other active treatments or placebo (mean age, 9.1 to 9.9 years). The LDX treatment arms had a slightly higher mean proportion of females (28%) than the mean for all treatments (range, 17% to 25%). Models that were unadjusted and then adjusted for covariates (see NMA covariate effects) were used to assess the impacts of these baseline differences among treatment groups.
Table 1. Summary of trial and baseline patient characteristics, by treatment.
NMA results
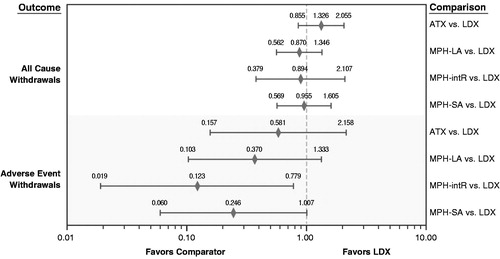
Relative treatment effects of each included treatment versus LDX are presented for each efficacy endpoint () and safety endpoint (). For the efficacy endpoints in comparisons among the active treatments, RR equates to a relative probability of a positive treatment response. A RR <1 favors LDX over the comparator. For the efficacy endpoints, available data were not sufficient to compare LDX and MPH-intR for the definition of ADHD-RS response that used 20%, 30%, or 40% as a proxy to 25% response. Similarly, available data were not sufficient to compare LDX with either MPH-intR or MPH-SA for the definition of ADHD-RS response that used only 25% response data. For the safety endpoints in comparisons among the active treatments, a RR >1 indicates a higher risk of withdrawal for patients taking the comparator treatment than for patients taking LDX. For all comparisons, results are considered statistically significant at the 5% level when the 95% confidence interval (CI) excludes 1.
Figure 3. Forest plot of network meta-analysis relative risks for treatment response based on ADHD-RS and CGI-I. ADHD-RS = ADHD Rating Scale; ATX = atomoxetine; CGI-ADHD-S = Clinical Global Impressions–ADHD–Severity; CGI-I = Clinical Global Impression–Improvement scale; LDX = lisdexamfetamine; MPH-LA = methylphenidate long acting; MPH-intR = methylphenidate intermediate release; MPH-SA = methylphenidate short acting.
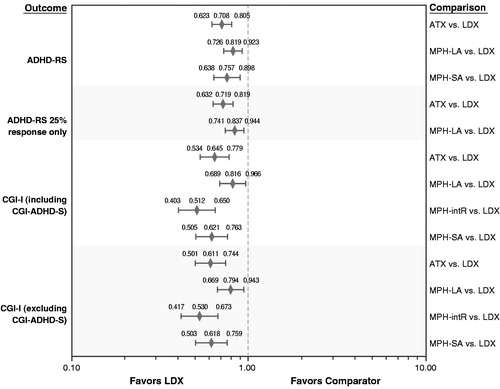
Figure 4. Forest plot of network meta-analysis relative risks for all-cause and adverse-event-related withdrawals. ATX = atomoxetine; LDX = lisdexamfetamine; MPH-LA = methylphenidate long acting; MPH-intR = methylphenidate intermediate release; MPH-SA = methylphenidate short acting.
For the ADHD-RS and CGI-I efficacy endpoints, LDX was statistically superior to all other active treatments for which comparison was possible. Specifically, the relative probabilities of response for ATX versus LDX were 0.71 (95% CI, 0.62–0.81; P < 0.001) for ADHD-RS and 0.65 (95% CI, 0.53–0.78; P < 0.001) for CGI-I; for MPH-LA versus LDX, they were 0.82 (95% CI, 0.73–0.92; P < 0.001) for ADHD-RS and 0.82 (95% CI, 0.69–0.97; P < 0.018) for CGI-I. The point estimates and statistical significance were maintained in the sensitivity analyses that excluded reductions other than 25% for the ADHD-RS response outcome and excluded CGI-ADHD-S responses for the CGI-I response outcome.
For the all-cause withdrawal safety endpoint, there were no significant differences between LDX and any of the other treatments. For withdrawal related to adverse events, MPH-intR had statistically fewer such withdrawals than LDX, and there were no other statistically significant differences.
NMA covariate effects
reports estimated covariate effects for age, sex, and baseline ADHD-RS score for three endpoints: ADHD-RS, CGI-I, and all-cause withdrawals. As noted previously, patients treated with LDX were on average older than patients treated with other treatments; however, in the covariate investigations, age was a significant covariate for only the ADHD-RS endpoint. For the age-adjusted model, increasing age was significantly associated with reduced ADHD-RS scores (covariate effect estimate: −0.303 [95% CI, −0.552 to −0.053; P = 0.018]), although upon inspection, the differences between the unadjusted and age-adjusted estimates of relative treatment effects were small.
Table 2. Effects of trial and patient characteristics used as covariates in network meta-analysis models.
Given the small differences between the unadjusted and covariate-adjusted model estimates, there was no compelling case to suggest that any of the covariate-adjusted models consistently improved on their unadjusted counterparts. Therefore, the main analyses presented here are for the unadjusted models.
NMA model fit
The reduced chi-square statistic (Pearson chi-square value divided by the remaining degrees of freedom) was used to assess and evaluate the fit of the NMA models with and without covariate terms. Reduced chi-square values close to 1 indicate the best fitting models. The reduced chi-square statistics for all endpoints are presented in .
Table 3. Reduced chi-squared statistics, by NMA model and inclusion or exclusion of covariates.
The reduced chi-square estimates for the efficacy endpoints, ADHD-RS and CGI-I, were close to 1, ranging from 0.86 to 1.53, indicating that the models fit the data well and accounted for most of the heterogeneity. Adding age as a covariate improved the fit of the NMA model for the ADHD-RS endpoint, but had little effect on fit for the other endpoints.
Reduced chi-square estimates for the safety endpoints, all-cause withdrawal and withdrawal due to adverse events were small and therefore of more concern. The low values could imply overestimated variability and therefore inflated widths of confidence intervals. The main factor contributing to these model-fit concerns was small cell counts.
Comparison of indirect evidence with head-to-head estimates
presents the relative treatment effect estimates calculated from head-to-head active treatment comparisons within a trial, where data were available, and those estimated in using adjusted indirect comparison methods. We performed these consistency checks for the endpoints ADHD-RS and CGI-I. Forest plots and calculations supporting these consistency checks are presented in the online supplement. For ADHD-RS and CGI-I, the adjusted indirect comparison RR point estimates and CIs closely matched results from the head-to-head trials. In all comparisons, the CI for the direct meta-analysis lay wholly within the CI for the adjusted indirect comparison, and the consistency check p values were all non-significant. In addition to the reduced chi-square values in , this finding supports confidence in the performance of the NMA network and model for the efficacy endpoints.
Table 4. Comparison of indirect evidence of NMA results with head-to-head estimates.
Heterogeneity tests for direct meta-analyses performed on placebo-controlled trials
presents the heterogeneity test P values for the direct meta-analyses performed on the placebo-controlled trials for the endpoints ADHD-RS and CGI-I. Forest plots detailing the direct meta-analyses are presented in the online supplement. There are some differences noted between results of the trials included in these direct meta-analyses, notably for LDX and MPH-LA versus placebo for CGI-I, and LDX, ATX, and MPH-LA versus placebo for ADHD-RS. We considered analyses excluding the trial(s) that appeared to contribute most to the statistical heterogeneity. However, in the absence of obvious reasons to do so, we did not pursue exclusion, and the NMAs presented are, if anything, conservative estimates for LDX.
Table 5. Heterogeneity test results for direct meta-analyses of placebo-controlled trials.
Discussion
The results from this evidence synthesis provide a valuable contribution to the ADHD literature. This information will enable clinicians to evaluate all relevant published evidence comparing LDX with MPH and ATX as the basis of their clinical decision-making process. In addition, this information will aid formulary decision makers in making coverage decisions with economic implications since the results from this evidence synthesis will be used as data inputs for cost-effectiveness analyses in ADHD. Subsequently, we anticipate that with better available data, informed decisions can be made with an appropriate balance between health benefits and costs when selecting the most appropriate interventions in ADHD.
It is important to emphasize that meta-analyses like those presented in this manuscript complement, rather than replace, results from good-quality, head-to-head trials. However, as illustrated here, network meta-analyses can and do play an important role by enabling indirect treatment comparisons for treatments that have not been compared directly within a trial.
This systematic review and meta-analysis met the thorough standards required by health technology assessment agencies. Through comprehensive literature searches and screening of identified publications, we identified an evidence base of information describing the current pharmacological treatment options available for children and adolescents with ADHD in Europe and the relative impacts of these treatments. A major focus was on how the treatment of interest, LDX, compares with other active treatment options, in terms of both efficacy and safety.
LDX has been licensed for use in the United States, Canada, and Brazil as a treatment for ADHD in children and adolescents (the Food and Drug Administration has also approved LDX for use in adults in the United States). It is a recently approved treatment option in Europe, and a previous meta-analysisCitation20 has suggested that LDX may have better efficacy than MPH, which is currently recommended under the NICE guidanceCitation13.
The NMA results reported here indicate that when compared with ATX, MPH-LA, and MPH-SA, a higher proportion of patients on LDX achieved a positive treatment response for the efficacy endpoints. For ADHD-RS-defined responders, we estimated LDX to have 41% more responders than ATX, 22% more responders than MPH-LA, and 32% more responders than MPH-SA. For CGI-I-defined responders, we estimated that LDX had 55% more responders than ATX, 23% more responders than MPH-LA, 95% more responders than MPH-intR, and 61% more responders than MPH-SA. Both ADHD-RS and CGI-I are common scales used to measure treatment response in clinical research and clinical practice. The results of the NMA show that LDX demonstrated better efficacy than the comparators, regardless of whether a rating scale (ADHD-RS) or a more holistic clinical assessment of response (CGI-I) was used. These data may help clinicians make treatment decisions that allow early treatment adoption and optimal treatment management of ADHD. These results may also be useful for researchers designing future comparative clinical studies.
Although the analyses reported here also estimated more withdrawals for adverse events with LDX than with ATX, MPH-LA, MPH-intR or MPH-SA, these estimates should be interpreted with caution given the poor fit of the NMA model of this endpoint. The large residual variance and therefore large CIs for this endpoint suggest a lack of precision in the point estimates, which is a consequence of the small number of withdrawals due to adverse events that were observed within the trials. However, results presented here clearly support the superior efficacy of LDX relative to other currently accepted treatments for ADHD in children and adolescents.
Further investigation of the inconsistency of relative treatment effects observed in head-to-head trials and NMA results for the withdrawal endpoints suggested that a likely reason for these differences is the inconsistent direction of treatment effects across trials. For example, there is contradictory evidence for the comparisons of ATX and LDX versus placebo for the all-cause withdrawal endpoint. Six studies reported ATX as having a lower risk of all-cause withdrawal than placebo, in contrast to the other nine trials comparing these treatmentsCitation27,Citation49–53. Two studies reported placebo as having a higher risk of withdrawal than LDXCitation17,Citation19, and one study reported placebo as having a lower risk of withdrawal than LDXCitation18. When analyzing all-cause withdrawals, it is important to consider that heterogeneity can be inevitable due to study design and protocol requirements; for example, rules for withdrawal due to lack of efficacy or tolerability can differ from trial to trial, and some trials may have follow-on trials allowing patients to ‘drop out’ into an open-label follow-on study.
Inconsistencies were present for withdrawal due to adverse events, although to a lesser degree. The point estimates for LDX versus MPH-LA were similar, but those for ATX versus MPH-LA were inconsistent. Similar but less pronounced inconsistencies in direction of treatment effect were observed for this endpoint between placebo-controlled trials involving ATX and MPH-LA. For the Kratochvil et al.Citation53 trial, a greater risk of withdrawal was observed in the placebo arm than in the ATX arm, in contrast to all other trials that included ATX and placebo. No withdrawals due to adverse events were observed in either arm of the Montoya et al.Citation54 trial. The Findling et al.Citation55 trial showed a higher risk of withdrawal due to adverse events in the MPH-LA arm than in the placebo arm, in contrast to the Wilens et al.Citation56 and CoghillCitation17 trials, which showed higher risk in the placebo arm.
Lacking evidence to exclude any of the contradictory trials, the NMA aims to consolidate differences in treatment effects as much as possible. It may be preferable to defer to the head-to-head trial results in situations such as this, where the NMA results are inconsistent.
The consistency checks of direct meta-analysis and head-to-head data with indirect evidence assessed the internal validity of this NMA. The lack of any statistical differences between direct and indirect evidence supports the internal validity of this NMA, but we acknowledge that these tests lack statistical power. Assessing our results for external validity with a previously published meta-analysisCitation20, we find that although the results are not strictly comparable in that the previous meta-analysis reported effect sizes (versus placebo) and did not perform indirect comparisons, the greater treatment effects were noted for amphetamine treatments rather than methylphenidate, which is consistent with our findings.
As with all meta-analyses, certain limitations should be considered when interpreting or using the results of this meta-analysis. All clinical trials included in this meta-analysis investigated the short-term efficacy of ADHD medications in patients with ADHD without comorbid disorders, which contrasts with daily clinical practice wherein treatment is long term and patients show high rates of comorbid disorders. In addition, clinical trial settings do not reflect real-world practice in terms of medication compliance. A recent study showed that LDX-treated patients demonstrated better treatment adherence compared with patients initiated on other ADHD medications, except for MPH-LA and ATX, in treatment-naïve children and adolescentsCitation57. The data sources and the specific endpoints used are important limitations for results presented here. Each clinical trial had inclusion criteria that defined the population represented by the clinical trial sample. When the clinical trials are combined, and particularly when the samples differ for the various endpoints, it is not clear what population(s) are represented. It is conceivable that the meta-analytic samples represent the union of the populations represented in each trial. It is equally conceivable that the meta-analytic samples represent the intersection of the populations represented. For example, consider two clinical trials with different age requirements: 5 to 10 years for the first trial and 8 to 16 years for the second trial. When the meta-analysis is performed for these two clinical trials, it is debatable whether the combined meta-analysis sample represents persons aged 5 to 16 years (union) or those aged 8 to 10 years (intersection).
Analyses of trial heterogeneity identified some between-trial differences in inclusion and exclusion criteria that could influence the meta-analytic results in unmeasured ways. For example, although all trials selected here studied patients diagnosed with ADHD, some of the selected trials also allowed participation of patients with comorbid oppositional defiant disorder, one of the most common psychiatric comorbidities seen with ADHDCitation6. Trials that specifically studied patients with other comorbid conditions (depression, anxiety, autism, and tic disorders) were excluded from analyses conducted here. However, additional comorbid conditions observed in patients but not defined as inclusion or exclusion criteria within the trial could further confound and limit meta-analytic conclusions.
Analyses of covariate effects identified differences between treatment populations that were evident across the selected trials. The most notable differences were that patients in the LDX trials were on average 2 years older than those in the other trials, and the LDX trials had approximately 5% more females than the other trials. When these parameters were included as covariates in the NMA models, there was little consistent effect on the estimates from the indirect treatment comparisons. For this reason, the primary analyses reported here did not include covariates. It is worth noting that in meta-analytic approaches, lack of significant covariates does not imply lack of relationship between measured covariates and the outcomes or treatments. The presence of covariate relationships cannot be formally investigated in trial-level data analyses; covariate relations can be verified only in patient-level data analyses. Obviously, the potential for unmeasured patient-level differences is another factor to consider when thinking about the representativeness of the meta-analytic results reported here. One important factor that could affect treatment response is dose selection. Unfortunately, we were unable to investigate the effect of fixed versus optimized dosing strategies as this factor was collinear with the study and therefore could not be included in the analyses.
Important study design features differed across the trials included in this meta-analysis. As one illustration, the studies included here used different endpoint definitions for ADHD-RS response: 25% reductionCitation19, 30% reductionCitation56, 40% reductionCitation49. Heterogeneity will characterize any meta-analytic samples that use different study design parameters. The impacts of design-related heterogeneity on meta-analytic results can be more or less obvious, depending on the particular trials included and the individual impacts of alternative design parameters. In analyses presented here, outstanding heterogeneity-related questions pertained to the definitions of adverse events requiring withdrawal within each trial and the appearance of a large placebo effect in one of the LDX trialsCitation18.
Another potential limitation is that of the analytical methods selected within the published articles. For the efficacy response outcomes, the last observation carried forward approach was used more often than not when patients discontinued prior to study completion. The limitations of such analytical methods are well known, but the implications of applying them in any given analysis are more difficult to ascertain. It is of some reassurance that this approach was adopted fairly consistently across the different treatment types.
One additional limitation is that the analyses reported here made no statistical adjustments for multiple comparisons within these meta-analyses. Given the number of treatment comparisons and outcomes, one might expect to see significant results by chance. However, this limitation is tempered somewhat by the reduced precision in NMAs and indirect comparison analyses, which make statistically significant results hard to achieve.
Conclusion
This research systematically collated and synthesized the currently available evidence, yielding our conclusion that LDX is an efficacious treatment option available for physicians in prescribing treatments for children and adolescents with ADHD.
Transparency
Declaration of funding
This study was funded by Shire. Shire develops and markets psychiatric drugs including treatments for ADHD.
Author contributions: N.R. planned, performed and interpreted the meta-analyses. E.Z., P.H., and J.S. designed the research question and contributed to the content and review of all aspects of the systematic review, meta-analyses, and reporting. All authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, are fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development.
Declaration of financial or other relationships
RTI Health Solutions (RTI-HS) was paid as a consultant to Shire for this study. N.R. and E.Z. have disclosed that at the time of this research and analysis they were employees of RTI-HS. N.R. has disclosed that he is now an employee of BresMed Health Solutions. J.S. is an employee of Shire and holds stock/or stock options. P.H. was a full-time employee of Shire and held stock/or stock options when this work was conducted. He is now an employee of Vertex Pharmaceuticals.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Supplementary Material
Download PDF (1.2 MB)Acknowledgments
The authors thank Miny Samuel, RTI Health Solutions, for her contributions regarding the design and conduct of the literature searches and systematic review. Haim Erder from Shire and Moshe Fridman from AMF Consultancy also reviewed and edited the manuscript for scientific accuracy.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Arlington: American Psychiatric Publishing, 2000
- Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and meta-regression analysis. Am J Psychiatry 2007;164:942-8
- Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children – United States, 2003 and 2007. Morb Mortal Wkly Rep 2010;59:1439-43
- Castells X, Ramos-Quiroga JA, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2011;6:CD007813
- Management of attention deficit and hyperkinetic disorders in children and young people: a national clinical guideline. Scottish Intercollegiate Guidelines Network, 2009. Available at: http://www.sign.ac.uk/guidelines/fulltext/112/index.html [Last accessed 7 March 2014]
- Coghill D, Soutullo C, d’Aubuisson C, et al. Impact of attention-deficit/hyperactivity disorder on the patient and family: results from a European survey. Child Adolesc Psychiatry Ment Health 2008;2:31
- Biederman J, Wilens TE, Mick E, et al. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry 1998;44:269-73
- DiScala C, Lescohier I, Barthel M, et al. Injuries to children with attention deficit hyperactivity disorder. Pediatrics 1998;102:1415-21
- Jensen PS, Shervette RE 3rd, Xenakis SN, et al. Psychosocial and medical histories of stimulant-treated children. J Am Acad Child Adolesc Psychiatry 1988;27:798-801
- Pomerleau OF, Downey KK, Stelson FW, et al. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse 1995;7:373-8
- Milberger S, Biederman J, Faraone SV, et al. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36:37-44
- Danckaerts M, Sonuga-Barke EJ, Banaschewski T, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry 2010;19:83-105
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. NICE Clinical Guideline 72. September 2008. Available at: http://www.nice.org.uk/nicemedia/pdf/CG072NiceGuidelineV2.pdf [Last accessed 7 March 2014]
- Methylphenidate, atomoxetine and dexamfetamine for attention deficit hyperactivity disorder (ADHD) in children and adolescents [TA98]. National Institute for Health and Clinical Excellence, 2006. Available at: http://www.nice.org.uk/TA98 [Last accessed 7 March 2014]
- Banaschewski T, Coghill D, Santosh P, et al. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry 2006;15:476-95
- Lisdexamfetamine [package insert]. Available at: http://www.medicines.org.uk/emc/medicine/27442/SPC/Elvanse+30mg%2c+50mg+%26+70mg+Capsules%2c+hard/#PHARMACOKINETIC_PROPS [Last accessed 2 January 2014]
- Coghill D, Banaschewski T, Lecendreux M, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 2013;23:1208-18
- Findling RL, Childress AC, Cutler AJ, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011;50:395-405
- Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 2007;29:450-63
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry 2010;19:353-64
- Single technology appraisal: specification for manufacturer/sponsor submission of evidence. National Institute for Health and Care Excellence, June 2012. Available at: http://www.nice.org.uk/media/D54/6E/SpecificationForManufacturerSponsorSubmissionOfEvidenceJune2012.doc [Last accessed 3 December 2013]
- DuPaul GJ, Power TJ, Anastopoulos AD, et al. The ADHD Rating Scale-IV: Checklist, Norms, and Clinical Interpretation. New York: Guilford Press, 1998
- Faries DE, Yalcin I, Harder D, et al. Validation of the ADHD Rating Scale as a clinician administered and scored instrument. J Atten Disord 2001;5:107-15
- Guy W (ed.) Clinical Global Impressions (CGI). In: ECDEU Assessment Manual for Psychopharmacology. Rockville: US Department of Health and Human Services, Public Health Service, Alcohol Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, 1976:218-22
- Whitehead A. Meta-analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons Ltd, 2002
- Jones B, Roger J, Lane PW, et al; PSI Health Technology Special Interest Group, Evidence Synthesis sub-team. Statistical approaches for conducting network meta-analysis in drug development. Pharm Stat 2011;10:523-31
- Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2002;63:1140-7
- CRD’s guidance for undertaking reviews in health care. Centre for Reviews and Dissemination, 2009. Available at: http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf [Last accessed 7 March 2014]
- Sharp WS, Walter JM, Marsh WL, et al. ADHD in girls: clinical comparability of a research sample. J Am Acad Child Adolesc Psychiatry 1999;38:40-7
- Abikoff HB, Vitiello B, Riddle MA, et al. Methylphenidate effects on functional outcomes in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). J Child Adolesc Psychopharmacol 2007;17:581-92
- Bangs ME, Hazell P, Danckaerts M, et al. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics 2008;121:e314-20
- Biederman J, Quinn D, Weiss M, et al. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs 2003;5:833-41
- Dell’Agnello G, Maschietto D, Bravaccio C, et al. Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled Italian study. Eur Neuropsychopharmacol 2009;19:822-34
- Findling RL, Quinn D, Hatch SJ, et al. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry 2006;15:450-9
- Gau SS, Shen HY, Soong WT, Gau CS. An open-label, randomized, active-controlled equivalent trial of osmotic release oral system methylphenidate in children with attention-deficit/hyperactivity disorder in Taiwan. J Child Adolesc Psychopharmacol 2006;16:441-55
- Greenhill LL, Findling RL, Swanson JM; ADHD Study Group. A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 2002;109:E39
- Kemner JE, Starr HL, Ciccone PE, et al. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther 2005;22:498-512
- Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84
- Martenyi F, Zavadenko NN, Jarkova NB, et al. Atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry 2010;19:57-66
- Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159:1896-901
- Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose–response study. Pediatrics 2001;108:E83
- Pliszka SR, Browne RG, Olvera RL, et al. A double-blind, placebo-controlled study of Adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2000;39:619-26
- Steele M, Weiss M, Swanson J, et al. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can J Clin Pharmacol 2006;13:e50-62
- Takahashi M, Takita Y, Yamazaki K, et al. A randomized, double-blind, placebo-controlled study of atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2009;19:341-50
- Wang Y, Zheng Y, Du Y, et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry 2007;41:222-30
- Weiss M, Tannock R, Kratochvil C, et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005;44:647-55
- Wigal S, Swanson JM, Feifel D, et al. A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2004;43:1406-14
- Wolraich ML, Greenhill LL, Pelham W, et al. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics 2001;108:883-92
- Newcorn JH, Kratochvil CJ, Allen AJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 2008;165:721-30
- Block SL, Kelsey D, Coury D, et al. Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr (Phila) 2009;48:723-33
- Gau SS, Huang YS, Soong WT, et al. A randomized, double-blind, placebo-controlled clinical trial on once-daily atomoxetine in Taiwanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2007;17:447-60
- Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 2004;114:e1-8
- Kratochvil CJ, Vaughan BS, Stoner JA, et al. A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics 2011;127:e862-8
- Montoya A, Hervas A, Cardo E, et al. Evaluation of atomoxetine for first-line treatment of newly diagnosed, treatment-naive children and adolescents with attention deficit/hyperactivity disorder. Curr Med Res Opin 2009;25:2745-54
- Findling RL, Bukstein OG, Melmed RD, et al. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2008;69:149-59
- Wilens TE, McBurnett K, Bukstein O, et al. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 2006;160:82-90
- Setyawan J, Hodgkins P, Guérin A, et al. Comparing treatment adherence of lisdexamfetamine and other medications for the treatment of attention deficit/hyperactivity disorder: a retrospective analysis. J Med Econ 2013;16:962-75