Abstract
Objective:
The development of new formulations of extended-release (ER) opioids with abuse-deterrent technology attempts to deter prescription opioid abuse while maintaining appropriate access to care for pain patients. This study examined the degree to which some patients may avoid switching to reformulated ER opioids with abuse-deterrent technology and the extent to which those patients are more likely to be abusers.
Research design and methods:
We analyzed Truven MarketScan pharmacy and medical claims data following the introduction of two reformulated ER opioids with abuse-deterrent technology. Adults aged 18–64 who were continuous users of extended-release oxycodone HCl (ER oxycodone) or extended-release oxymorphone HCl (ER oxymorphone) in a 6 month period prior to the introduction of the respective reformulations of those products were identified and categorized based on whether they switched to the reformulation, switched to other ER/long-acting (LA) opioids (without abuse-deterrent technology), or discontinued ER/LA opioid treatment in a 6 month post-reformulation period. Abusers were identified using ICD-9-CM diagnosis codes for opioid abuse/dependence. Pearson’s chi-squared tests and Fisher’s exact tests were then used to compare rates of abuse between patients who avoided switching to a reformulated ER opioid. Sensitivity analyses examined several definitions used in this analysis.
Main outcome measures:
ER/LA opioid utilization; rates of diagnosed opioid abuse.
Results:
A total of 31%–50% of patients avoided switching to reformulated ER opioids. Rates of diagnosed opioid abuse were higher among these patients compared to patients who transitioned to the reformulated ER opioids.
Limitations:
Due to the observational research design, caution is warranted in causal interpretation of the findings. The study was conducted among commercially insured continuous ER oxycodone or ER oxymorphone users; future research should consider additional patient populations, such as non-continuous users and those without commercial insurance (i.e., Medicare, Medicaid, uninsured).
Conclusions:
Some patients switched to other ER/LA opioids without abuse-deterrent technology or discontinued ER/LA opioid treatment when their existing ER treatment was reformulated. Rates of opioid abuse were higher among patients who switched to other ER/LA opioids or discontinued ER/LA opioid treatment, suggesting that abusers may seek more easily abuseable alternatives such as prescription opioids without abuse-deterrent technology.
Introduction
The abuse of prescription opioids (RxOs) is a major public health problem in the US, and some have even classified the problem as an ‘epidemic’Citation1. In 2012, an estimated 2.1 million Americans aged 12 or older (or 0.8% of that population) had pain reliever dependence or abuseCitation2. Abuse of prescription drugs can be as dangerous as the use of illegal drugs, leading to addiction and even deathCitation3. RxOs accounted for more than 15,500 deaths in 2009, nearly a four-fold increase compared to 1999Citation4, which amounts to more overdose deaths than those attributed to heroin and cocaine combinedCitation5. At the same time, pain is a common condition and cause of disability in the USCitation6. The Food and Drug Administration (FDA) has acknowledged that RxOs are an important component of modern pain managementCitation7, and the utilization of RxOs has continued. These trends highlight the difficult balance associated with RxOs, as noted by the FDA: “on the one hand, providing access to pain medications for those who need them, and on the other hand, managing the variety of risks posed by analgesic drugs”Citation8.
Numerous public and private efforts have attempted to address the problem of RxO abuse. For example, the Prescription Drug Abuse Prevention Plan from the Office of National Drug Control Policy recommends actions in four major areas to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcementCitation3.
Another approach that attempts to deter RxO abuse while maintaining appropriate access to care for pain patients has been the development of new formulations of extended-release (ER) opioids with characteristics designed to resist crushing and deter abuse. ER opioids provide a longer period of drug release and can thus be taken less frequently than immediate-release/short-acting (IR/SA) opioids, but their ER characteristics may be overcome by manipulation or tampering, and some abusers may find ER opioids to be more attractive than IR opioids due to their higher drug dosages. Reformulated ER opioids with abuse-deterrent technology are designed to be more difficult to crush, break, dissolve, or inject.
In April 2013, the FDA determined that reformulated ER oxycodone HCl (reformulated ER oxycodone) has abuse-deterrent properties designed to make certain types of abuse more difficult and approved abuse-deterrent labeling for the productCitation9. Reformulated ER oxymorphone HCl (reformulated ER oxymorphone) was “developed to incorporate technology designed to render the tablet highly resistant to crushing without affecting its extended-release properties”, but the FDA determined in May 2013 that the post-marketing investigations supporting an abuse-deterrent claim were inconclusiveCitation10. The FDA determined that “[w]hile there is an increased ability of the reformulated version of [ER oxymorphone] to resist crushing relative to the original formulation, study data show that the reformulated version’s extended-release features can be compromised when subjected to other forms of manipulation”Citation11.
Recent research has raised concerns that in the presence of a reformulated ER opioid with abuse-deterrent technology, some patients being treated for substance abuse may seek alternative targets for abuse, possibly undercutting the potential benefits of these reformulated ER opioidsCitation12,Citation13. To date, little is known about the effect of reformulated ER opioids with abuse-deterrent technology in a commercially insured population, namely the degree to which some patients may avoid switching to reformulated ER opioids, and the extent to which patients who avoid switching to these reformulated ER opioids are more likely to be abusers. The aim of this study was to assess patient behavior following the introduction of two reformulated ER opioids with abuse-deterrent technology, focusing on a commercially insured patient population. Specifically, the study examined the degree to which some patients may avoid switching to reformulated ER opioids with abuse-deterrent technology and the extent to which those patients are more likely to be abusers.
Methods
Using 2010–2012 Truven MarketScan de-identified pharmacy and medical claims data for commercially insured patients, we analyzed ER/long-acting (LA) opioid utilization patterns and rates of diagnosed opioid abuse in the period surrounding the introduction of reformulated ER oxycodone with abuse-deterrent technology in August 2010. The reformulation of ER oxycodone may have motivated some ER oxycodone patients who were looking for abuseable drugs to switch to other RxOs without abuse-deterrent technology. The introduction of reformulated ER oxycodone along with the discontinuation of the original formulation provided an opportunity to assess patient behavior in response to this change in an observational, real-world setting.
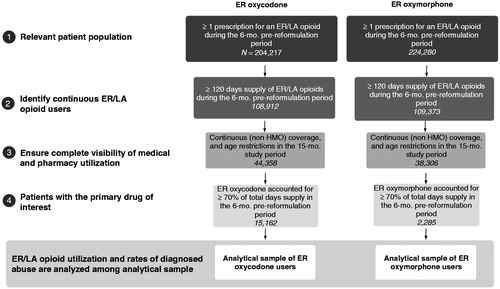
We restricted our sample to commercially insured patients, aged 18–64, with continuous use of ER/LA opioids (at least 120 days’ supply of ER/LA opioids). We required that patients’ primary ER/LA opioid in the 6 month period from February to August 2010 (pre-reformulation period) was ER oxycodone. The primary ER/LA opioid was defined as the ER/LA opioid that accounted for at least 70% of the days’ supply of all of their ER/LA opioids in a 6 month period. The 70% criterion was based on expert physician input, and we examined alternative thresholds of 60% and 80% in sensitivity analyses. Our rationale was to focus the analysis on patients who were using ER oxycodone consistently in the pre-reformulation period. We then observed these patients’ ER/LA opioid utilization in a 6 month period from November 2010 to May 2011 (post-reformulation period), allowing for a 3 month transition period between the pre- and post-reformulation periods.
We examined whether ER oxycodone patients switched to reformulated ER oxycodone with abuse-deterrent technology, switched to other ER/LA opioids without abuse-deterrent technology, or discontinued ER/LA opioid treatment altogether in the 6 month post-reformuation period (i.e., the 6 month period following the introduction of reformulated ER oxycodone with abuse-deterrent technology, as defined above). Similar to the criterion used in the pre-reformulation period, patients were categorized based on their primary ER/LA opioid in the post-reformulation period (i.e., the ER/LA opioid that accounted for at least 70% of the days’ supply of all of their ER/LA opioids in that 6 month period) in order to address the fact that pain patients often switch RxOs (opioid rotation) or use different RxOs concomitantly. Patients could also have discontinued ER/LA opioid treatment altogether. Among those patients who discontinued ER/LA opioid treatment in the post-reformulation period, we further segmented patients based on whether they used IR/SA opioids or discontinued use of any IR/SA or ER/LA opioids in the post-reformulation period.
We then evaluated whether ER oxycodone patients who avoided switching to reformulated ER oxycodone with abuse-deterrent technology had higher rates of opioid abuse than patients who switched to reformulated ER oxycodone. A patient was classified as an opioid abuser if he or she had any medical claims associated with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for opioid abuse or dependence (305.5x, 304.0x, 304.7x, 965.00, 965.02, and 965.09) during the study period which spanned the pre- and post-reformulation periods. Pearson’s chi-squared tests were used to compare rates of diagnosed opioid abuse between groups of patients. Alternative time periods over which abuse diagnoses could occur and alternative definitions of opioid abuse (i.e., separating ICD-9-CM diagnosis codes for opioid abuse and dependence) were examined in sensitivity analyses, and they yielded consistent patterns (see below). A similar analysis was conducted for reformulated ER oxymorphone which was introduced in March 2012 and also uses technology designed to deter abuse. Due to a substantially smaller sample of ER oxymorphone users, Fisher’s exact tests were used instead of Person’s chi-squared tests to compare rates of diagnosed opioid abuse between groups of patients.
Results
ER/LA opioid utilization patterns
Study inclusion/exclusion criteria were met by 15,162 continuous users of ER oxycodone and 2285 continuous users of ER oxymorphone (). When faced with a reformulation of their existing ER opioid treatment – in essence, a forced switch – the majority of ER oxycodone patients transitioned to reformulated ER oxycodone with abuse-deterrent technology. As shown in , Panel A, among ER oxycodone patients, 69.4% switched to reformulated ER oxycodone. However, a substantial portion switched to other ER/LA opioids without abuse-deterrent technology (21.3%) or discontinued ER/LA opioid treatment (9.3%). Most patients (76.0%) who discontinued ER/LA opioid treatment in the post-reformulation period used IR/SA opioids in the post-reformulation period, though some (24.0%) discontinued use of any IR/SA or ER/LA opioids.
Table 1. Opioid utilization patterns and differences in rates of diagnosed opioid abuse following the introductions of reformulated ER oxycodone and ER oxymorphone.
A similar analysis of reformulated ER oxymorphone yielded results that were directionally consistent. Among ER oxymorphone patients, 50.3% switched to reformulated ER oxymorphone, 6.9% switched to reformulated ER oxycodone, 25.4% switched to other ER/LA opioids without abuse-deterrent technology, and 17.4% discontinued ER/LA opioid treatment in the post-reformulation period (, Panel B). Among those who discontinued ER/LA opioid treatment in the post-reformulation period, most (82.7%) used IR/SA opioids in the post-reformulation period, though some (17.3%) discontinued use of any IR/SA or ER/LA opioids.
These findings are consistent with changes in the total volume of ER/LA opioid prescriptions at the aggregate level in the US, as observed in IMS National Prescription Audit data for the period 2010–2012. We analyzed changes in the volume of ER/LA opioid prescriptions following the introduction of reformulated ER oxycodone and, separately, following the introduction of reformulated ER oxymorphone by comparing prescription volume in the quarter prior to the introduction of a reformulated product to prescription volume in the same quarter the following year, post-reformulation. In other words, for ER oxycodone, we compared prescription volume in the second quarter of 2010 to prescription volume in the second quarter of 2011; for ER oxymorphone, we compared prescription volume in the fourth quarter of 2011 to prescription volume in the fourth quarter of 2012. The prescription volume of ER oxycodone decreased by 20.5% following the introduction of reformulated ER oxycodone while all other ER/LA opioids experienced an increase in prescription volume over the same period (results available upon request). The prescription volume of ER oxymorphone decreased by 32.0% following the introduction of reformulated ER oxymorphone while most other ER/LA opioids experienced an increase in prescription volume over the same period.
Differences in rates of diagnosed abuse
In addition to the aforementioned observed shifts away from reformulated ER oxycodone and reformulated ER oxymorphone at both the aggregate and patient levels, there were higher rates of diagnosed opioid abuse among those who switched to other ER/LA opioids without abuse-deterrent technology or who discontinued ER/LA opioid treatment after the introduction of these reformulated ER/LA opioids. The rate of diagnosed opioid abuse among ER oxycodone patients who switched to other ER/LA opioids without abuse-deterrent technology was higher than that of patients who switched to reformulated ER oxycodone (6.7% vs. 3.5%, relative risk 1.9, p < 0.001) (, Panel A). Similarly, ER oxycodone patients who discontinued ER/LA opioid treatment following the introduction of reformulated ER oxycodone had a higher rate of diagnosed opioid abuse compared with patients who switched to reformulated ER oxycodone (10.9% vs. 3.5%, relative risk 3.1, p < 0.001). Among the ER oxycodone patients who discontinued ER/LA opioid treatment in the post-reformulation period, the highest rate of diagnosed opioid abuse (11.3%) occurred among those who used IR/SA opioids in the post-reformulation period, and this rate was 3.2 times that of patients who switched to reformulated ER oxycodone.
A similar pattern was observed for reformulated ER oxymorphone. The rate of opioid abuse among ER oxymorphone patients who switched to other ER/LA opioids without abuse-deterrent technology appeared slightly higher than that of patients who switched to reformulated ER oxymorphone, though the difference was not statistically significant (2.6% vs. 2.1%, relative risk 1.2, p = 0.498), and the rate was similar to that of patients who switched to reformulated ER oxycodone (2.5%) (, Panel B). ER oxymorphone patients who discontinued ER/LA opioid treatment had a higher rate of opioid abuse than patients who switched to reformulated ER oxymorphone (5.0% vs. 2.1%, relative risk 2.4, p = 0.004). Among the ER oxymorphone patients who discontinued ER/LA opioid treatment in the post-reformulation period, the highest rate of diagnosed opioid abuse (7.2%) occurred among those who discontinued IR/SA or ER/LA opioids in the post-reformulation period, and this rate was 3.5 times that of patients who switched to reformulated ER oxycodone.
These findings suggest that abusers may seek more easily abuseable alternatives such as RxOs without abuse-deterrent technology, which is consistent with a 2013 study of individuals assessed for substance abuse treatmentCitation12. Some abusers may also switch to illicit substances such as heroinCitation13. These studies focused on individuals being treated for substance abuse; our study examined a broader patient population and found that patients who avoided switching to reformulated ER opioids were more likely to be diagnosed with opioid abuse or dependence.
Sensitivity analyses
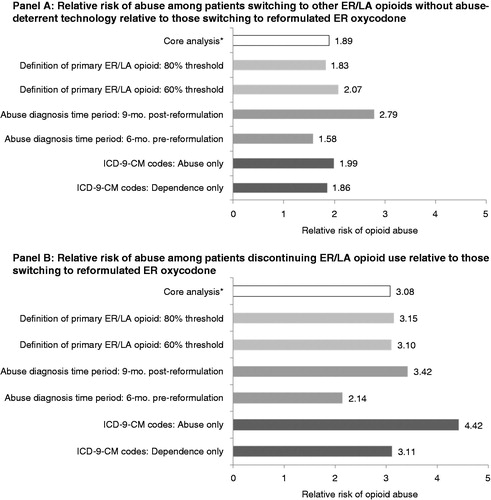
We conducted several sensitivity analyses. First, we examined whether the 70% threshold used to define a patient’s primary ER/LA opioid had a meaningful impact on the results by considering alternative thresholds of 60% and 80%. When a 60% threshold was used to define a patient’s primary ER/LA opioid, there was a larger starting sample of ER oxycodone patients and ER oxymorphone patients, and a greater share of those patients switched to the reformulated products (). The relative differences in abuse rates were similar to those from our core analysis. When an 80% threshold was used to define a patient’s primary ER/LA opioid, there was a smaller starting sample of ER oxycodone patients and ER oxymorphone patients, and a smaller share of those patients switched to the reformulated products (). As was the case with the 60% threshold, the relative differences in abuse rates were similar to those from our core analysis. ER/LA utilization patterns and differences in rates of diagnosed opioid abuse following the introductions of reformulated ER oxycodone and ER oxymorphone did not differ in a meaningful way when alternative thresholds were used to define a patient’s primary ER/LA opioid.
Table 2. Sensitivity analysis: 60% threshold for defining a patient’s primary ER/LA opioid (opioid utilization patterns and differences in rates of diagnosed opioid abuse following the introductions of reformulated ER oxycodone and ER oxymorphone).
Table 3. Sensitivity analysis: 80% threshold for defining a patient’s primary ER/LA opioid (opioid utilization patterns and differences in rates of diagnosed opioid abuse following the introductions of reformulated ER oxycodone and ER oxymorphone).
For the sensitivity analyses that follow, we focused on ER oxycodone patients, as there was a much smaller sample of ER oxymorphone patients. We examined whether the use of different definitions of opioid abuse altered our results. We limited the analysis to the ICD-9-CM diagnosis codes for opioid abuse only (excluding ICD-9-CM diagnosis codes for opioid dependence). The absolute rates of abuse using this narrower definition of abuse were lower, as would be expected, but the relative risk of being an abuser among those who switched to ER/LA opioids without abuse-deterrent technology compared to those who switched to reformulated ER oxycodone (relative risk of 2.0, p value <0.001 using abuse-only diagnosis codes) (, Panel A) was similar to that in our core analysis (relative risk of 1.9, p value <0.001 using both abuse and dependence diagnosis codes) (, Panel A). The relative risk of being an abuser among those discontinuing ER opioid use compared to those who switched to reformulated ER oxycodone was higher with the use of abuse-only diagnosis codes (relative risk of 4.4, p value <0.001) than with the use of both abuse and dependence diagnosis codes (relative risk of 3.1, p value <0.001). When dependence-only ICD-9-CM diagnosis codes were used (excluding ICD-9-CM diagnosis codes for opioid abuse), the relative risks yielded a consistent pattern. This sensitivity analysis suggests that ICD-9-CM diagnosis codes for opioid abuse and dependence may be used interchangeably, as one would not expect that a reformulated ER opioid with abuse-deterrent technology would directly impact dependence. In addition, a prior study on the excess costs of opioid abuse found that the magnitude of the excess costs was similar regardless of whether abuse-only or dependence-only ICD-9-CM diagnosis codes were usedCitation14.
Table 4. Sensitivity analyses: alternative definitions of opioid abuse and alternative time periods over which abuse diagnoses could occur (differences in rates of diagnosed opioid abuse following the introductions of reformulated ER oxycodone and ER oxymorphone).
As another sensitivity analysis, we examined whether alternative time periods over which abuse diagnoses could occur affected the findings. In the main analysis, the full 15 month study period (6 month pre-reformulation period, 3 month transition period, 6 month post-reformulation period) was used to calculate rates of opioid abuse, as shorter time periods would reduce the likelihood of capturing interactions between opioid abusers and medical professionals resulting in an abuse-related medical claim. Regardless of whether the 6 month pre-reformulation period or the 9 month period post-reformulation (including the 3 month transition period and the 6 month post-reformulation period) was used, there were higher rates of opioid abuse among patients who switched away from reformulated ER oxycodone and either switched to other ER/LA opioids without abuse-deterrent technology or discontinued ER/LA opioid treatment (, Panel B). The absolute rates of abuse were lower for these shorter time periods compared to the rate of abuse for the full 15 month study period, as would be expected since shorter time periods would be less likely to capture interactions between opioid abusers and medical professionals resulting in an abuse-related medical claim, as mentioned above. The relative risk of being an abuser among those who switched to other ER/LA opioids without abuse-deterrent technology compared to those who switched to reformulated ER oxycodone ranged from 1.6 to 2.1 (all p values <0.001). The relative risk of being an abuser among those discontinuing ER/LA opioid use compared to those who switched to reformulated ER oxycodone ranged from 2.8 to 3.4 (all p values <0.001). summarizes the relative risk of abuse across all sensitivity analyses conducted for ER oxycodone: Panel A summarizes the relative risk of abuse among patients switching to other ER/LA opioids without abuse-deterrent technology relative to those switching to reformulated ER oxycodone, and Panel B summarizes the relative risk of abuse among patients discontinuing ER/LA opioid use relative to those switching to reformulated ER oxycodone.
Figure 2. Sensitivity analyses for ER oxycodone patients. *The core analysis uses a 70% threshold for the definition of a primary ER/LA opioid, a 15 month time period for abuse diagnosis, and both abuse and dependence ICD-9-CM diagnosis codes. p Values were <0.001 for all relative risks shown above.
Discussion
This study finds 31% of ER oxycodone patients and 50% of ER oxymorphone patients avoided switching to the reformulated versions of their existing ER opioid treatment which used abuse-deterrent technology. These patients switched to other ER/LA opioids without abuse-deterrent technology or discontinued ER/LA opioid treatment altogether. Rates of opioid abuse were higher among patients who switched to other ER/LA opioids or discontinued ER/LA opioid treatment.
A recent FDA press announcement noted that “[t]he development of abuse-deterrent opioid analgesics is a public health priority for the FDA”Citation9. Reformulated ER opioids with abuse-deterrent technology represent an important step towards reducing RxO abuse while maintaining appropriate access to care for pain patients. However, potential unintended consequences exist when addressing a complex problem such as RxO abuse. This research suggests that the introduction of reformulated ER opioids with abuse-deterrent technology may result in substitution to more abuseable alternatives such as other RxOs without abuse-deterrent technology. This finding corroborates an earlier study on abuse rates and routes of administration of reformulated ER oxycodone which noted “the ease with which users can currently switch to non-tamper-resistant products”Citation12.
Addressing RxO abuse will likely require a comprehensive approach that combines investments in substance abuse treatment for RxO abusers who may be prone to substitution to alternative substances, programs to monitor use of RxOs, and new technologies that hinder abuse of RxOs. Intentional substitution away from reformulated RxOs with abuse-deterrent technology may serve as a signal of potential abuse. As new RxOs with abuse-deterrent properties continue to be introduced, studies should be undertaken to examine potential unintended consequences such as changes in RxO utilization and differences in rates of opioid abuse.
This study has several limitations. First, due to the observational research design used in the claims data analyses, caution is warranted in any causal interpretation of the findings. While the introduction of reformulated ER oxycodone and reformulated ER oxymorphone allows for a quasi-experimental study design, it is not a full substitute for a true randomized experiment. Therefore, while we observed changes in ER/LA opioid utilization and differences in rates of diagnosed opioid abuse by ER/LA opioid utilization patterns associated with the introduction of reformulated ER oxycodone and reformulated ER oxymorphone, it is still possible that other factors may have contributed to these changes. For example, one might be concerned that changes in formulary status or co-payments may have caused some patients to switch away from ER oxycodone or ER oxymorphone around the time the reformulated products were introduced. We were unable to observe changes in formulary status in the claims data, but we were able to examine changes in average co-payments per prescription for ER oxycodone and ER oxymorphone before and after the introduction of the reformulation products. We found no meaningful increases in average co-payments per prescription during this period and even observed declines in average co-payments per prescription in some cases (results available upon request). Therefore, we believe that the observed changes in ER/LA opioid utilization were not attributed to changes in co-payments.
Second, our analysis was conducted among continuous ER oxycodone or ER oxymorphone users so we were unable to examine changes in ER/LA opioid utilization and differences in rates of diagnosed opioid abuse by ER/LA opioid utilization patterns among individuals who used ER oxycodone or ER oxymorphone sporadically. Future research should examine the impact of reformulated ER oxycodone or ER oxymorphone in the broader population of patients, part of which may not use ER oxycodone or ER oxymorphone continuously or at all. Recent studies have found that while reformulated ER oxycodone is aimed at deterring abuse, serious abusers may find other substances to abuse, such as ER/LA or IR/SA opioids without abuse-deterrent properties or illicit drugs such as heroin. Future research should also examine the potential unintended consequences of reformulated ER oxycodone or ER oxymorphone such as their impact on the abuse of drugs without abuse-deterrent characteristics, as determined abusers may switch to other more abuseable drugs.
Third, we were only able to identify abusers who were diagnosed for their abuse. Prior research has found that a significant share of opioid abuse is not formally diagnosedCitation15. We were unable to identify undiagnosed opioid abusers in our claims database, so it is unclear whether our results would generalize to undiagnosed opioid abusers. Future research should examine the impact of RxOs with abuse-deterrent technology on individuals abusing RxOs but not yet formally diagnosed with opioid abuse, as it would shed light on potential public health impacts of RxOs with abuse-deterrent technology in the broader population.
Finally, this study uses Truven MarketScan claims data for a large sample of commercially insured individuals throughout the US. Future research should examine the impact of RxOs with abuse-deterrent technology on Medicare and Medicaid beneficiaries and the uninsured.
Conclusion
Following the introduction of reformulated ER opioids with abuse-deterrent technology, some patients avoided switching to the reformulated version of their existing ER opioid treatment and instead switched to other ER/LA opioids without abuse-deterrent technology or discontinued ER/LA opioid treatment. Rates of opioid abuse were higher among patients who switched to other ER/LA opioids or discontinued ER/LA opioid treatment, suggesting that abusers may seek more easily abuseable alternatives such as prescription opioids without abuse-deterrent technology. Reformulated ER opioids with abuse-deterrent technology represent a promising approach towards reducing RxO abuse while maintaining appropriate access to care for pain patients, but providers and policymakers should be aware that there may be potential unintended consequences to reformulated ER opioids, such as a shift towards more easily abuseable alternatives.
Transparency
Declaration of funding
This study was funded by Purdue Pharma LP.
Declaration of financial/other relationships
R.B.-J. has disclosed that he is an employee of Purdue Pharma LP. N.Y.K., A.S., and H.G.B. have disclosed that they are employees of Analysis Group Inc., a consulting company that received funding for this research from Purdue Pharma LP. E.M. has disclosed that he is a consultant on this project and received funding from Analysis Group.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial or other relationships to disclose.
Acknowledgments
The authors gratefully acknowledge the contributions of Katharine Bodnar and Michael Kaminsky.
Previous presentation: Some of the material contained in this paper was presented at the Academy of Managed Care Pharmacy’s 26th Annual Meeting & Expo, Tampa, FL, 1–4 April 2014.
References
- Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control. CDC. Prescription painkiller overdoses. November 2011. Available at: http://www.cdc.gov/HomeandRecreationalSafety/pdf/PolicyImpact-PrescriptionPainkillerOD.pdf [Last accessed November 2013]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. Available at: http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.pdf [Last accessed September 2013]
- Office of National Drug Control Policy. Epidemic: Responding to America’s prescription drug abuse crisis. 2011. Available at: http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf [Last accessed March 2014]
- U.S. Food and Drug Administration. FDA introduces new safety measures for extended-release and long-acting opioid medications. 2012. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310870.htm [Last accessed January 2013]
- Vital signs: overdoses of prescription opioid pain relievers – United States, 1999–2008. Morb Mortal Wkly Rep 2011;60:1487-92
- Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003;290:2443-54
- U.S. Food and Drug Administration. Guidance for Industry. Abuse-Deterrent Opioids – Evaluation and Labeling. Draft Guidance. 2013. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM334743.pdf [Last accessed February 2013]
- Woodcock J. A difficult balance – pain management, drug safety, and the FDA. N Engl J Med 2009;361:2105-7
- FDA approves abuse-deterrent labeling for reformulated OxyContin. Silver Spring, MD: U.S. Food and Drug Administration, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm348252.htm [Last accessed 16 August 2013]
- Alexander L, Mannion RO, Weingarten B, et al. Development and impact of prescription opioid abuse deterrent formulation technologies. Drug Alcohol Depend 2014: published online 16 February 2014, doi:10.1016/j.drugalcdep.2014.02.006
- FDA Statement: Original Opana ER Relisting Determination. Silver Spring, MD: U.S. Food and Drug Administration, 2013. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm351357.htm [Last accessed 4 February 2014]
- Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain 2013;14:351-8
- Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med 2012;367:187-9
- White AG, Birnbaum HG, Mareva MN, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm 2005;11:469-79
- White AG, Birnbaum HG, Rothman DB, et al. Development of a budget-impact model to quantify potential cost savings from prescription opioids designed to deter abuse or ease of extraction. Appl Health Econ Health Policy 2009;7:61-70