Re: Baker DL et al. Evaluation of two commercial omalizumab/free IgE immunoassays: implications of use during therapy. Curr Med Res Opin 2014;30:913-22
Dear Editor,
We have read your articleCitation1 and we would like to comment on it. The article describes the in-house testing (at Genentech) of two commercial kits for monitoring free IgE and omalizumab in the case of antibody therapy with omalizumab (Xolair).
You confirm that in cases in which treatment with the anti-IgE antibody omalizumab has been unsuccessful, the need for therapy monitoring has arisen. However, you advise against therapy monitoring (free IgE and omalizumab) and refer to the official omalizumab-dosing table, although said dosing table is not up to date – at least for Europe.
For measuring free IgE with omalizumab therapy, you compared the BioTeZ recovery ELISA assay and the ViraCor-IBT free IgE assay with Genentech in-house free IgE ELISA assay and the omalizumab assay. Only the BioTeZ recovery ELISA assay is commercially available; other assays are not available for individual measurements.
The ‘in vivo samples’ used in the article are obviously pooled patient sera and not native individual samples. In your investigation of these samples, you show that the functional capability and quality of commercial assays is insufficient and that the levels of free IgE identified may be too high. You conclude that using these measurements to monitor therapy would result in an overdose. We only want to mention that in contrast to your results, in our clinical samples, we found low levels of free IgE and higher levels of omalizumab. It must be stated that the recovery ELISA IgE/omalizumab assay is a multiplex assay, which provides additional information about drug activity (omalizumab activity)Citation2.
In contrast to other assays tested by the authors, the BioTeZ recovery IgE ELISA/omalizumab assay is a quantitative method that simultaneously provides three measurements for each sample: the concentration of free IgE, the degree of IgE neutralization (activity), and the concentration of therapeutic antibody omalizumab still available. The IgE neutralization ratio indicates how much IgE was bound in the serum by therapeutic antibodies – the ‘recovery’ refers to the recovery of the IgE added to the sample as a reciprocal of the degree of neutralisationCitation2.
shows the recovery of IgE in relation to the concentration of the therapeutic antibody. shows the degree of neutralization of IgE (omalizumab activity) or the ability of the omalizumab IgE to bind as a function of the omalizumab level. is at least the inverse function of Citation2,Citation3. The functions describe the interaction between the IgE and omalizumab biomolecules. The curve shows that the relative ability of omalizumab to neutralize the IgE decreases with increasing dose.
Figure 1. (a) Recovery of IgE in relation to the concentration of the therapeutic antibody. (b) Degree of neutralization of IgE (omalizumab activity) or ability of the omalizumab IgE to bind as a function of the omalizumab level.
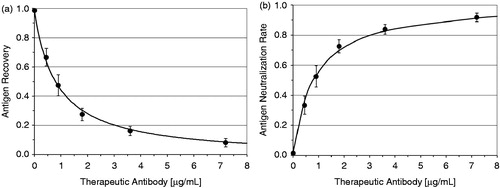
The BioTeZ recovery ELISA IgE/omalizumab assay is currently the only commercial measurement method that reflects the direct interaction between IgE and omalizumab. This parameter is ignored by the authors.
We are convinced that a parameter of the drug activity can prevent over- or under-dosage in asthma, urticaria, or psoriasis therapies with omalizumab – namely when it is seen in which IgE neutralization area a patient is and where it is apparent that additional IgE neutralization can be expected from a change in the dose.
In you will find an example of an analysis of a serum sample that was measured with the recovery ELISA IgE/omalizumab assayCitation5. Doctors can match this information with data from the patient record, e.g. applied dose, application intervals, and body weight.
Table 1. Example of a measurement result from one serum sample.
Table 2. Stability of equilibriumCitation4.
The authors believe that the level of free IgE measured by the recovery ELISA assay was too high and suspect that this results from long incubation times and the high sample dilution. For incubation, it should be noted that although the omalizumab-IgE complex quickly reaches equilibrium in solution, binding to the solid phase is merely delayed and occurs after 16 h. The binding kinetics of IgE omalizumab complex to the capture antibody coated on the surface of a microplate with different IgE concentrations will be stable after 16 hours of incubation time. The stability of optical density (O.D.) was published by Strohner et al. in 2013Citation4. Stability is given between 16 and 24 hours of incubation ().
In order to measure two analytes (IgE and omalizumab) in parallel with the recovery ELISA assay, both analytes must be in a measurable range. For this reason, native serum samples are measured in a dilution of 1:20. In the recovery ELISA assay for IgE and omalizumab, the measurement ranges were optimized by measuring native samples from patients undergoing omalizumab therapy and adjusting them to the clinically relevant range. The ranges of both analytes (IgE: 2.4–1210 ng/ml, omalizumab: 400–80,000 ng/ml) significantly differ from those of the Genentech in-house methods for which a significantly lower measurement range is given for both free IgE (2–150 ng/ml) and omalizumab (≥28 ng/ml). The Genentech omalizumab assay is also diluted 1:100.
As the authors appropriately write, comparing ELISAs with widely varying ranges as well as with different precision profiles and error distributions is always a source of deviations, which need to be analyzed. We see no excessive source of error in the dilution selected during the recovery ELISA assay and are amazed at the poor reproducibility.
We have determined the inter-assay variance (), which is an important quality criterion of the assay. For this purpose, five native human serum samples were selected. Four of the five samples were from patients undergoing omalizumab therapy. These data are available in the user manualCitation5.
Table 3. Inter-assay variance of recovery ELISA IgE/omalizumabCitation5.
We are deeply concerned by the reproducibility and false positives determined by the authors, which contradict our own quality management based measurements. Our considerations range from errors caused by improper assay execution to methodological issues. In this context, we also question why the software developed to evaluate the recovery IgE ELISA/omalizumab assay was not mentioned by the authors. This software already includes a quality control and provides information on the accuracy of the experimental measurements.
A probable cause is the in vitro modification of the samples as well as the handling of the samples between analyses 1 and 2, which is not explained by the authors. It is critical that both different omalizumab-positive samples (in vivo samples) and omalizumab-negative samples from different patients were pooled with a native IgE level of 50–700 IU/ml and spiked with different amounts of omalizumab (in vitro samples). The term ‘in vivo samples’ appears to be misleading.
It is assumed that IgE binds with omalizumab and that this binding process is crucially influenced by a number of factors such as incubation time, temperature, and the initial concentration of the components. Even when pooling native omalizumab-positive samples, there should be binding reactions after mixing, depending on the sample composition and how much unbound omalizumab was included in the samples. The authors did not provide any further information about the mixing conditions of the samples or any supplements. We would also like to note that results have not been presented for reproducibility with the other assays.
In Table 4 of the authors’ article, it would have been informative to show the measured values of the other assays, too. That could possibly explain why the authors’ Figure 3 shows levels of free IgE that are above the measuring range of the Genentech assay.
With respect to the target level of <50 ng/ml IgE mentioned by the authors and the maximum allowed pre-treatment IgE level of 30–700 IU/ml, reference is made to current dosing recommendations for pre-treatment IgE up to 1500 IU/ml IgE ( in reference 6)Citation6. The publication on the comparison of different diagnostics is cause for discussion.
Why should the desire of doctors for more information in case of treatment failure not be justified?
Does personalized medicine not also entail a personalization of the dose?
Is the level of free IgE during omalizumab therapy not relevant, although it is the target molecule and has a limit value <50 ng/ml, or it is because the therapeutic concentrations are saturated?
In our opinion, sound results can only be achieved through experiments with a transparent framework. Unlike the authors, we believe that the BioTeZ recovery IgE ELISA/omalizumab assay is suitable for reliably measuring serum samples from patients during omalizumab therapy. Although we have not yet had any reasons to doubt this, we will conduct further tests.
Unfortunately, we cannot check the methods and results presented in the article because the assays and samples are not available. We are available for further discussions and investigations. We strongly welcome diagnostic methods that are suitable for personalized medicine, as we believe that this trend is becoming increasingly important – not only in the case of non-responders.
In addition to the development of highly innovative biologicals, the associated diagnostics should also be encouraged. BioTeZ is willing to meet this challenge and is always ready to cooperate.
Transparency
Declaration of funding
No funding for this letter was provided.
Declaration of financial/other relationships
P.S. has disclosed that he is the inventor and patent holder of recovery ELISA. G.B. has disclosed that he is an independent medical expert and reviewer for approval as a medical device for equipment and diagnostic tools, e.g. for recovery ELISA.
Notes
*Xolair is a registered trademark of Novartis, East Hanover, NJ, USA
References
- Baker DL, Peng K, Cheu M, Fischer SK. Evaluation of two commercial omalizumab/free IgE immunoassays: implications of use during therapy. Curr Med Res Opin 2014;30:913-22
- Strohner P. Patent: EP 06828568.3. Immunoassay for the simultaneous immunochemical determination of an analyte (antigen) and a treatment antibody targeting the analyte in samples (recovery immunoassay). AT454629 (T) -2010-01-15 European Patent Office
- Strohner P, Staatz A, Sarrach D, et al. The recovery ELISA – a newly developed immunoassay for measurement of therapeutic antibodies and the target antigen during antibody therapy. Clin Chem Lab Med 2012;50:1263-9
- Strohner P, Korn S, Buhl R, Becher G. The recovery-ELISA – a novel assay technique to monitor therapy with humanized antibodies: the example of omalizumab. J Immunoassay Immunochemistry 2013;34:83-93
- BioTeZ Berlin-Buch GmbH: RIO user manual – instructions for enzyme immunoassay for the quantitative in-vitro determination of free IgE and available therapeutic antibody omalizumab in human serum samples. Available at: http://www.biotez.de/index.php/de/unternehmen/downloads.html [Version 4, Last accessed 6 May 2014]
- Document WC 500 057 298 from EMA – Annex I – Summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000606/WC500057298.pdf [Last accessed 4 April 2014]

