Dear Editor
We are writing this letter in response to the comments made by Becher and Strohner on our paper ‘Evaluation of two commercial omalizumab/free IgE immunoassays: implications of use during therapy’.
We would like to reiterate that the purpose of this paper is to discourage the use of free IgE to adjust omalizumab dosing, which is considered off-label use of omalizumab. The manuscript is meant to demonstrate that measurement of free ligands (in this case IgE) is technically very challenging and different methods can produce very different results. This paper is not suggesting that the Genentech assay is the gold standard, rather that the Genentech free IgE assay is the clinically relevant assay in this context because it was used to generate the FDA approved omalizumab dosing table and the target free IgE levels. Regardless of the inconsistencies observed with the BioTeZ assay, both commercial assays tend to have over-recovery compared with the Genentech assay. Therefore, we felt compelled to warn against the use of commercial free IgE assays for off-label use as the two commercial assays produce very different results compared with the Genentech assay. The data was only to show how different the IgE results can be and the potential risk for unnecessary treatment and/or risk to patient safety.
There are specific comments made that we would like to address:
For measuring free IgE with omalizumab therapy, you compared the BioTeZ recovery ELISA assay and the ViraCor-IBT free IgE assay with Genentech in-house free IgE ELISA assay and the omalizumab assay. Only the BioTeZ recovery ELISA assay is commercially available; other assays are not available for individual measurements.
This point was made in the paper. We did mention that this assay is no longer available. The ViraCor-IBT free IgE assay was available at the time this evaluation was performed. In fact, the sample evaluations were actually conducted by ViraCor-IBT.
The ‘in vivo samples’ used in the article are obviously pooled patient sera and not native individual samples. Omalizumab-positive samples (in vivo samples) and omalizumab-negative samples from different patients were pooled with a native IgE level of 50–700 IU/ml and spiked with different amounts of omalizumab (in vitro samples). The term ‘in vivo samples’ appears to be misleading. Even when pooling native omalizumab-positive samples, there should be binding reactions after mixing, depending on the sample composition and how much unbound omalizumab was included in the samples.
To ensure a wide range of IgE and omalizumab levels for this study, both in-vivo and in-vitro serum sample sets were utilized. The 20 in-vivo samples were prepared by combining serum from allergic asthma patients with similar free IgE and omalizumab profiles from an omalizumab clinical trial to generate samples with a wide range of free IgE and omalizumab. The clinical samples were combined to protect patient identity as well as provide sufficient sample volume for the various assays. The 36 in-vitro samples were prepared by adding varying amounts of omalizumab to omalizumab-naïve allergic asthma serum samples based on the total IgE levels to ensure that a good range of free IgE and omalizumab was represented. Both the in-vivo and in-vitro samples were given ample time for mixing and equilibration. The samples were then aliquoted and submitted for various assay evaluations including omalizumab levels and free IgE levels in the Genentech assay. All the assays used the same samples. The aim was to evaluate the assays not an individual’s omalizumab/free IgE levels.
We only want to mention that in contrast to your results, in our clinical samples, we found low levels of free IgE and higher levels of omalizumab. It must be stated that the recovery ELISA IgE/omalizumab assay is a multiplex assay, which provides additional information about drug activity (omalizumab activity).
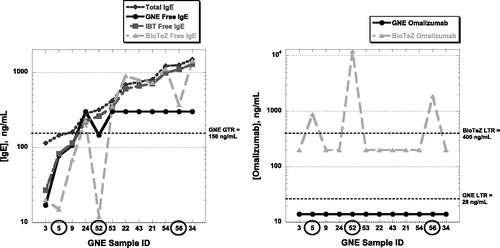
As illustrated in the paper Figure 4, the BioTeZ assay falsely detected omalizumab in three samples where no omalizumab was present. The free IgE levels detected in these same three samples was significantly lower in the BioTeZ assay than either the ViraCor-IBT or Genentech free IgE assays. The correlation between omalizumab and free IgE levels obtained from the BioTeZ assay does not seem to be informative for these samples ().
In order to measure two analytes (IgE and omalizumab) in parallel with the recovery ELISA assay, both analytes must be in a measurable range. For this reason, native serum samples are measured in a dilution of 1:20. The Genentech omalizumab assay is also diluted 1:100.
In our experience high dilution of samples can lead to dissociation of complexes and over-recovery of analyte of interest when measuring free analyte levels. This is why we have minimized dilution of samples to 1:2 in the Genentech free IgE assay.
The statement that the omalizumab assay is diluted 1:100 is correct. The omalizumab assay is designed to quantify total omalizumab levels so the dilution step actually promotes dissociation of omalizumab/IgE complexes in samples and helps to minimize the interference from IgE.
We are deeply concerned by the reproducibility and false positives determined by the authors which contradict our own quality management based measurements. Our considerations range from errors caused by improper assay execution to methodological issues.
BioTeZ offers kits to interested parties for sample analysis. Genentech made several attempts to contact BioTeZ through their website for sample evaluations, without receiving a response from the company. However, evaluation of samples through use of the kits is an option that BioTeZ offers. The sample evaluations were conducted using BioTeZ kits at a third party contract research organization (CRO) who is very familiar with evaluation of various samples and kits. The same CRO was used to generate the omalizumab and free IgE results using Genentech assays.
In this context, we also question why the software developed to evaluate the recovery IgE ELISA/omalizumab assay was not mentioned by the authors. This software already includes a quality control and provides information on the accuracy of the experimental measurements.
The CRO that evaluated the samples did use the software provided by BioTeZ. The recoveryELISA kit contains quality control samples and calibrators, which are analyzed in duplicates and at different concentrations. The acceptance criteria are not explicitly outlined in manufacturer’s manual. All results are entered in an evaluation spreadsheet as part of the evaluation tool provided by the manufacturer, and based on the final calculation the assay run was judged as valid or invalid. All results reported were for assays that were valid based on the manufacturer’s specifications.
We would also like to note that results have not been presented for reproducibility with the other assays.
The re-evaluation of the same samples was done only with the BioTeZ method as some of the values were very different from the Genentech and Viracor-IBT methods. This was done to ensure there were no issues with the analysis of the first set of samples. During this process, however, we noticed that the values for a large number of samples were very different from the first run, therefore we concluded that the method is not reproducible.
Also, we have good confidence in the reproducibility of the Genentech methods. Based on validation experiments performed at a third party CRO the omalizumab free IgE inter-assay variance for the controls ranged from 6.0% to 9.4%.
Why should the desire of doctors for more information in case of treatment failure not be justified? Does personalized medicine not also entail a personalization of the dose? Is the level of free IgE during omalizumab therapy not relevant, although it is the target molecule and has a limit value <50 ng/ml, or it is because the therapeutic concentrations are saturated?
As mentioned in the paper, there are clearly multiple factors that should be considered in addition to the absolute free IgE levels of patients, as they can impact the efficacy of omalizumab in non-responders. Given the variability in the disease and mechanisms of non-responsiveness to omalizumab, achieving the same target free IgE level for every individual may not be adequate to improve the overall response rate to omalizumab treatment. Moreover, it is important to note that the omalizumab dosing table was developed based on data measured by the free IgE assay developed by Genentech. The dosing table is a very personalized way to treat patients and is based on weight and on total IgE at baseline; total IgE levels are elevated during omalizumab treatment due to circulating IgE–omalizumab complexes. Accordingly re-testing total IgE levels cannot be used to determine omalizumab doses (Genentech and Novartis US, 2013).
It should, however, be acknowledged that some patients do not respond to omalizumab and there are many potential underlying factors affecting response other than free IgE levels. As specified by the prescribing information, the most effective method for evaluating continued need for omalizumab is still periodic assessment of the patient’s disease severity and level of asthma control (Genentech and Novartis US, 2013).
Figure 1. a) A set of 12 omalizumab-naïve samples with various IgE levels was utilized for total and free IgE measurements using Genentech or the two commercial assays. Compared with the free IgE results obtained using the Genentech and ViraCor-IBT assays, 3 samples (#5, #52 and #56) under recovered significantly in the BioTeZ assay. (b) All 12 samples were omalizumabnaïve and therefore should not have measurable levels of omalizumab. However, the same 3 samples (#5, #52 and #56) yielded measurable false-positive levels of omalizumab in the BioTeZ omalizumab assay.
Transparency
Declaration of funding
This letter was not funded.
Declaration of financial/other relationships
D.L.B., K.P., M.C., and S.K.F. have disclosed that they are employees of Genentech Inc., South San Francisco, CA, USA.
References
- Genentech, Novartis US. Xolair FDA Prescribing Information. Available at: http://www.gene.com/gene/products/information/pdf/xolair-prescribing.pdf [Last accessed 25 November 2013]

