Abstract
Background:
Vascular endothelial growth factor (VEGF) inhibitors, including targeted therapy with tyrosine kinase inhibitors (TKIs) and the angiogenesis inhibitor bevacizumab, and mammalian target of rapamycin (mTOR) inhibitors are now the standard of care for metastatic renal cell carcinoma (mRCC). However, real-world treatment patterns are not well characterized.
Objective:
To describe treatment patterns during the first, second, and third lines of targeted therapies for mRCC among community oncologists in the US.
Methods:
Participating physicians recruited from a nationwide panel each identified up to 15 adult mRCC patients who initiated a second therapy after January 2010. Information extracted from medical records included types of targeted therapies, reasons for treatment choices, patterns of treatment discontinuation, and dose adjustments.
Results:
Thirty-six physicians contributed charts from 433 mRCC patients. Seventy-seven percent of patients received a VEGF inhibitor as first targeted therapy; 23% received an mTOR inhibitor. Among first-line VEGF users, second-line treatments were 66% mTOR and 34% VEGF inhibitors. Among first-line mTOR users, second-line treatments were 94% VEGF and 6% mTOR inhibitors. Sunitinib followed by everolimus was the most commonly used treatment sequence. Estimated median duration for second targeted therapy was 8.6 months, and median overall survival (OS) and progression-free survival (PFS) were 27.4 and 10.8 months, respectively. Efficacy, treatment guidelines and mechanism of action were the most important considerations for treatment choice.
Limitations:
Limitations include no adjustment for baseline characteristics, possible difference between physician-defined progression and central review in the clinical trial setting, and limited data availability for axitinib during the study period.
Conclusion:
In this large retrospective chart review among community oncologists, VEGF–mTOR–VEGF was the most common treatment sequence for mRCC. The most common drugs were sunitinib in the first line and everolimus in the second line.
Introduction
In 2012, over 60,000 new cases of renal cell carcinoma (RCC) and 10,000 RCC-related deaths are expected in the United States (US)Citation1. About one third of RCC cases will advance to metastatic diseaseCitation2. Seven novel therapies targeting the vascular endothelial growth factor (VEGF) or mammalian target of rapamycin (mTOR) pathways have been approved for the treatment of metastatic RCC (mRCC) since 2005. These agents include the tyrosine kinase inhibitors (TKIs) sorafenib, sunitinib, pazopanib, and axitinib; the antiangiogenic endothelial growth factor monoclonal antibody bevacizumab; and the mTOR inhibitors temsirolimus and everolimusCitation3. All of these new agents have demonstrated prolongation of progression-free survival (PFS) compared to interferon or placebo and have thus replaced cytokine therapy as the standard of care for mRCCCitation4–10.
Most mRCC patients develop resistance to their front-line targeted therapy within 6–11 months of starting treatmentCitation6,Citation11. Sequential lines of different targeted agents are typically required to maintain disease control. Currently, the optimal sequence of targeted therapies to treat mRCC has not been well defined by randomized trials, and is based largely on physician judgment. In the first-line setting, current clinical evidence suggests that patients with good or intermediate prognosis should receive VEGF-targeted agentsCitation4–7, whereas temsirolimus has shown benefit in poor prognosis patientsCitation8. In the second-line setting following TKI failure, the efficacy and safety of everolimus and axitinib has been established in two phase III randomized trials, the RECORD-1 and the AXIS trialsCitation9,Citation10. Currently clinical trial evidence comparing approved agents in the second-line setting is limited. In RECORD-1, everolimus prolonged progression-free survival compared to placeboCitation9 and in the AXIS trial axitinib was associated with prolonged progression-free survival compared to sorafenibCitation10. In the INTORSECT trial, comparing the effectiveness of sorafenib versus temsirolimus in the second-line treatment setting, no significant difference was noted between temsirolimus and sorafenib on progression-free survival but an overall survival advantage was reported for sorafenib in the second-line settingCitation12. In a parallel trial, no statistically significant difference was found between sorafenib and dovitinib in a third-line treatment settingCitation13. However, outcomes of second-line mTOR and TKI treated patients in the community setting have not been extensively explored. Data derived from oncology practitioners could help shed light on this topic.
Given the lack of definitive comparative evidence, no consensus has been established on the optimal treatment sequencing of therapies for mRCC. Several retrospective studies have described the real-world treatment patterns and outcomes of different second-line therapies following TKI failureCitation14–19. However, these studies focused on a subset of patient populations (e.g. first-line sunitinib-treated patients, second-line sorafenib-, temsirolimus-, and everolimus-treated patients) and did not capture the full spectrum of the practice patterns. The objective of the present study was to describe current practice patterns in treating mRCC, focusing on the selection of first- and second-line therapy, treatment sequences, reasons for treatment choices, treatment duration, dose adjustment, and treatment outcomes in order to provide insights into practice patterns and ideas for future studies.
Methods
Data source
A retrospective chart review and a parallel physician survey were conducted during May–June 2012 among community-based oncologists or hematologists in the US. Physicians from a nationwide oncology network (P4 Healthcare participating physicians) were screened and those with at least five mRCC patients under their care in 2011 were invited to participate. The physicians were blinded to the identity of the study sponsor and principal investigators at recruitment. The identity of the sponsor was revealed during a subsequently held live meeting where the study’s findings were presented (on an aggregate level). Physicians were reimbursed for the time spent participating in this study (time spent learning about the study, selecting eligible patient charts, and extracting and entering chart data). Each physician was asked to select up to 15 sequential patient charts meeting the study inclusion criteria: (1) aged 18 years or older with a confirmed diagnosis of mRCC; (2) initiated on second-line targeted therapy for the treatment of mRCC in January 2010 or later (to reflect more recent therapies and treatment patterns); and (3) the participating physician had access to the patient’s complete medical records related to RCC. No exclusion criteria were applied. A standardized chart abstraction form was developed by the study authors to collect patient information, including demographics, mRCC treatment and disease severity, types of first-, second-, and third-line therapies, reasons for the choice of therapy, dose adjustments, treatment discontinuation, and imaging test patterns. A survey was also developed, to be conducted in parallel for the same physicians, to collect physician-stated treatment preferences for first-, second-, and third-line therapies for mRCC patients, factors of consideration for the choice of each line of therapy, and frequency of using imaging tests for disease monitoring. To ensure interpretability, a pilot test was conducted with two physicians completing both the physician survey and the online chart abstraction form. No issues were found and full data collection was subsequently initiated. Neither the patients nor the physicians were identifiable to the investigators. Exemption from Institutional Review Board review was obtained from the New England Institutional Review Board.
Study measures
Treatment sequencing
The proportions of patients receiving different therapies as first-, second- and third-line treatments were summarized at the class level and at the individual drug level. In the present study, each line of treatment was defined as the use of a different therapy. Bevacizumab was grouped with TKIs (sunitinib, sorafenib, pazopanib, and axitinib) as a VEGF-targeted agent. Third-line treatment use was only summarized among patients who received an FDA approved agent. Treatment sequences were summarized for the overall patient sample, and for subgroups of patients with good-to-intermediate and poor Memorial Sloan-Kettering Cancer Center (MSKCC) risk at the time of first targeted therapy initiation. Physicians’ stated preferences for each line of treatment were also summarized by prognosis, i.e., for good-to-intermediate and poor prognosis patients, respectively.
Reasons for treatment choices
For the first- and second-line therapies, reasons for treatment choices were provided for each individual patient based on the chart data, including considerations such as treatment guidelines, efficacy of drug as demonstrated in randomized clinical trials (standard of care administration), physician experience, different mechanism of action, response to earlier lines of therapy, and patient preference. (A complete list is presented in the Results section.)
Treatment patterns
Modifications to second-line treatment were assessed using the patient chart data, including treatment discontinuation and dose adjustments, along with the reason for each modification. Median treatment duration (in months) since initiation of second-line therapy was summarized for the overall patient sample and for each second-line agent. Patients who didn’t have treatment discontinuation or death information reported were censored at their last available follow-up visit.
Treatment outcomes
Overall survival (OS) and progression-free survival (PFS) during second-line therapy were examined for all study patients. OS was defined as the time from initiation of the second line therapy to death from any cause. Patients were censored at the most recent contact. PFS was defined as the time from second-line therapy initiation to the earlier date of physician-assessed disease progression or death; patients were censored at the earlier date of treatment discontinuation or last follow-up visit. Assessment of progression was made by participating physicians based on worsening cancer-related symptoms or radiographic evidence.
Imaging test utilization patterns
The total number of imaging tests performed during second-line treatment as well as the date of testing, imaging modality, reason for imaging, and physician-reported use of RECIST for each imaging test were examined. Results on imaging test patterns will be reported separately.
Statistical analysis
Descriptive statistics were used to describe baseline characteristics, treatment sequencing, reasons for treatment choices, and treatment patterns. Means and standard deviations were reported for continuous variables, while frequencies and percentages were reported for categorical variables. Treatment duration for the second targeted therapy was calculated using the Kaplan–Meier (KM) method to account for censoring. All analyses were performed using SAS software version 9.2 (Cary, NC).
Results
Baseline characteristics
A total of 36 community-based physicians participated in the study. Among them, 86% had a dual practice in hematology and oncology, and the rest were medical oncologists. The majority (72%) were in practice for more than 10 years. Charts were reviewed for 433 mRCC patients who received a second targeted therapy in or after January 2010. summarizes the patient characteristics at the time of initiation of second targeted therapy. The patients had a mean age of 63 years and 64% were male. The average time from mRCC diagnosis to initiation of second targeted therapy initiation was 13 months. Almost half (46%) had metastatic diseases at the initial RCC diagnosis. Most patients (79%) had clear-cell histology, and the most common metastatic sites were lung (82%), lymph nodes (56%), and bone (55%). Most patients (88%) experienced disease progression while on the first-line therapy. At the time of initiating second targeted therapy, 52% patients had intermediate MSKCC risk and the rest were split between favorable (22%) and poor (25%) status.
Table 1. Patient characteristics at the initiation of second targeted therapy.
Treatment sequencing
The observed treatment sequences are summarized in . For first-line targeted therapy, 77% of the included patients received a VEGF inhibitor and the remainder, 23%, received an mTOR inhibitor. Sunitinib (67%) was the most common VEGF inhibitor and temsirolimus (94%) was the most common mTOR inhibitor in the first-line setting. Among the 334 patients receiving first-line VEGF inhibitors, 34% received VEGF and 66% received mTOR inhibitors in the second line. Pazopanib (35%) was the most common VEGF and everolimus (67%) was the most common mTOR inhibitor for second line following a first-line VEGF inhibitor. Among the 99 patients who received an mTOR inhibitor during first-line, 94% used a VEGF and 6% used another mTOR inhibitor in the second line. Sunitinib (40%) was the most commonly used second-line VEGF inhibitor and everolimus (100%) was the most commonly used second-line mTOR inhibitor following first-line mTOR. Overall, VEGF–mTOR (51%) was the most common treatment sequence for the first two lines, with sunitinib–everolimus (103 out of 433, 24%, numbers not directly reported in the figure) being the most commonly used sequence of first- and second-line therapies. Only 21% of the patients went on to receive a third-line therapy during the study period. Among these patients, VEGF–mTOR–VEGF (42%) was the most commonly observed treatment sequence, with sunitinib–everolimus–bevacizumab (6 out of 93, 6%) being the most frequently used sequence of therapies. Among all patients, sunitinib (52%), temsirolimus (21%), and sorafenib (12%) were the most frequently used first-line therapies, while everolimus was the most commonly used second targeted therapy (36%), followed by temsirolimus (17%), pazopanib (15%), and sunitinib (14%) ().
Figure 1. Observed treatment sequences. mTOR, mammalian target of rapamycin inhibitor; N, number; VEGF, vascular endothelial growth factor. 1. In this analysis, bevacizumab is grouped with the TKI class because it has the same therapeutic target as the TKI agents. 2. Out of a total of 433 patients in this study, only 91 initiated third targeted therapy. No patients treated with mTORs during first-line and second-line treatment received third-line therapy.
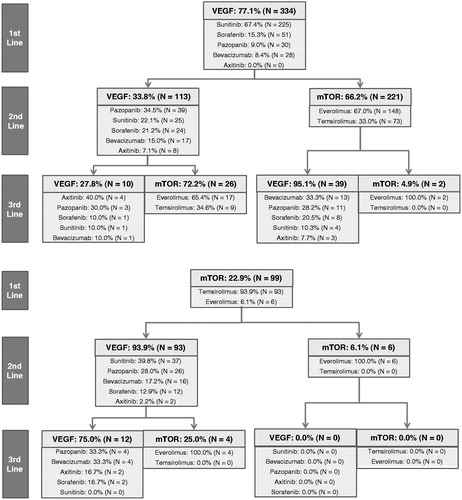
Table 2. Description of treatment patterns of second targeted therapy.
Physician-stated preferences for treatment sequences were also examined based on the parallel survey responses. For patients with good-to-intermediate prognosis, 79% of physicians preferred a VEGF and 21% preferred an mTOR inhibitor for first targeted therapy. Following a first-line VEGF inhibitor, 42% preferred a VEGF and 58% preferred an mTOR inhibitor as second-line therapy; following a first-line mTOR inhibitor, 83% preferred a VEGF and 17% preferred an mTOR inhibitor for second-line treatment. For patients with poor prognosis, 44% of the physicians reported preference for a VEGF inhibitor and 56% preferred an mTOR inhibitor for first targeted therapy. Following a first-line VEGF inhibitor, 43% preferred another VEGF inhibitor and 57% preferred an mTOR inhibitor as second targeted therapy; following a first-line mTOR inhibitor, 92% preferred a VEGF inhibitor and 8% preferred another mTOR inhibitor for second targeted therapy.
Reasons for treatment choices
The top five most important reasons for choice of a first targeted therapy for mRCC were efficacy of drug as demonstrated in randomized clinical trials (47%), treatment guidelines (33%), physician’s own experience with the drug (6%), route of administration (4%), and tolerability of drug (3%). For second targeted therapy, efficacy demonstrated in randomized trials (43%) remained the most important reason for therapy choice, followed by different mechanism of action from prior therapy (26%), treatment guidelines (12%), physician’s own experience with the drug (4%), and tolerability (4%), as the top five considerations for treatment choices.
Treatment patterns
The median follow-up time from the initiation of second-line therapy to the end of study follow up was 4.9 months, due to censoring as a result of reaching the time of chart review. During the follow-up period, 191 (44%) patients discontinued their second-line therapy, primarily due to disease progression (73%), drug toxicity/tolerability issues (9%), and death (9%). Discontinuation rates ranged from 38% to 61%, with everolimus-treated patients having the lowest rate, and sorafenib-treated patients the highest. Among patients who discontinued second-line treatment, the proportion of patients who discontinued due to disease progression ranged from 70% for sunitinib to 82% for bevacizumab across individual agents; and the percentage of patients who discontinued due to drug toxicity or intolerance ranged from 5% for everolimus to 18% for sorafenib ().
Among the patients who discontinued the second-line treatment, 50% received a third-line therapy, 31% died, and 16% received best supportive care. The estimated median duration of second-line therapy based on KM analysis was 8.6 months for all included patients, and varied between 4.4 months (bevacizumab) and 10.8 months (everolimus) across different therapies.
Twenty-seven percent of the patients received dose adjustments on second targeted therapy. Among them, 60% decreased total monthly dose, 25% had dose interruptions, and 19% had a drug holiday. Drug toxicity (70%) was the most common reason for dose adjustment, followed by disease progression (17%). When looking at individual therapies, dose adjustment rates varied between 20% and 53% for different second therapies. Sunitinib-treated patients had the highest dose adjustment rate, while dose adjustment rates were similar among other second-line therapies.
Not surprisingly, only 10 patients received axitinib as a second-line therapy given its recent FDA approval (January 2012), with a median follow-up time of 2.6 months. These patients were included in the overall analysis, but axitinib-specific statistics were not presented in given the small sample size and short follow-up time.
Treatment outcomes
During the study period, 89 (21%) patients died and 147 (34%) patients experienced disease progression on second-line therapy. Median durations of OS and PFS after initiation of the second-line therapy were 27.4 months and 10.8 months, respectively.
By treatment sequence, patients who received a VEGF inhibitor for first line followed by an mTOR inhibitor for second line (n = 221) had a median follow-up of 4.9 months after second-line initiation, during which time 20% of patients died and 40% progressed or died while on second therapy (). Median OS was not reached (the last patient was censored at 28.5 months; 51.8% patients remained alive at the last censoring date), and median PFS was 10.8 months after initiation of second targeted therapy. For patients who received VEGF–VEGF for first and second targeted therapies (n = 113), median follow-up was 6.1 months from initiation of second targeted therapy, during which time 21% died and 45% progressed or died. Median OS was 27.4 months, and median PFS was 10.3 months after initiating the second-line treatment. Patients who were treated with mTOR–VEGF for first and second therapies (n = 93) had a median follow-up of 4.9 months from initiation of second targeted therapy, during which 22% died and 40% progressed or died on second line. For these patients median OS was 23.6 months, and median PFS was 10.3 months following initiation of second-line treatment. Only six patients were treated with the mTOR–mTOR sequence.
Table 3. Treatment outcomes for mRCC patients sorted by different first- and second-line treatment sequences.
Subgroup analyses for treatment sequences
Using the patient chart data, patients were categorized into good-to-intermediate or poor risk groups based on their MSKCC score at the initiation of first targeted therapy. Eighty-five (19%) patients who did not have their MSKCC status reported in the data were not included in the subgroup analyses. The majority (91%) of good-to-intermediate risk patients received a VEGF inhibitor in the first-line setting, while the majority (65%) of poor risk patients received an mTOR inhibitor. Among patients who received first-line VEGF inhibition, more good-to-intermediate risk patients were treated with a second-line mTOR inhibitor compared to poor risk patients (68% vs. 59%). Among patients with a first-line mTOR inhibitor, the majority in both risk groups received second-line VEGF inhibition (100% and 94%). Results were similar when patients were categorized based on their MSKCC risk status at the initiation of second targeted therapy, with VEGF–mTOR–VEGF being the most common treatment sequence for the first three targeted therapies. However, among the poor risk patients at the initiation of second-line treatment, about half (55%) had received a VEGF inhibitor for first-line targeted therapy, and a large majority (70%) received an mTOR inhibitor for second line.
These observed treatment sequences based on patient chart data were compared with the preferred treatment sequences reported in the parallel physician survey data, stratified by severity group. The observed treatment sequences for patients with poor MSKCC stratum were largely consistent with physician-stated preferences for poor prognosis patients, except that observed use of mTOR inhibitors in the first-line setting was greater than the physician-reported estimates (65% vs. 56%). Observed treatment patterns differed from the physician reported preferences for patients with good-to-intermediate prognosis. Specifically, the patient data indicated greater actual use of mTOR inhibitors in the second line after a first line VEGF inhibitor (68% vs. 58%), as well as greater actual use of VEGF inhibitors in the first line (91% vs. 79%) and in the second line after a first-line mTOR inhibitor (100% vs. 83%).
Discussion
The present study captured current practice patterns in mRCC though retrospective review of medical records from community-based physicians. In this real-world setting, and at the time of this survey, we found that the most common sequence of targeted therapies was inhibition of VEGF–mTOR–VEGF. Sunitinib was the most commonly used first targeted therapy. Everolimus was the most commonly used second-line targeted therapy, regardless of first-line agent. Sunitinib followed by everolimus was the most frequently used sequence for the first two targeted therapies, while sunitinib–everolimus–bevacizumab was the most commonly observed treatment sequence for the first three lines. Primary stated reasons for the choice of targeted agents were efficacy as shown in clinical trial data (i.e. standard of care administration) and treatment guidelines, for both first- and second-line choices. Change in mechanism of action was a prominent consideration for choosing second targeted therapy, although this concept is still open to debate in the scientific community due to conflicting research dataCitation20,Citation21.
The estimated median duration for second targeted therapy in the present study was 8.6 months for all patients, and ranged from 4.4 to 10.8 months across individual therapies. These durations are consistent with findings from previous real-world studies of mRCC patientsCitation15,Citation22. However, they are longer than durations of second-line therapy observed in clinical trialsCitation9,Citation10. Differences in treatment durations could be due to differences in patient characteristics between clinical trial populations and real-world populations, and to differences in patient monitoring, as PFS in our study is ultimately a measure of time to treatment discontinuation and/or death rather than an objective measure of RECIST progression and/or death. It should also be noted that this chart review study had short lengths of follow-up time between the second-line treatment initiation and the time of chart review, and was not fully powered to assess second-line time-to-event outcomes. Future studies are warranted to shed light on the differences in outcomes between trials and real-world data sources in mRCC.
Percentages of patients who discontinued second targeted therapy due to drug toxicity were approximately twice as high among those receiving VEGF inhibitors compared to those receiving mTOR inhibitors for second targeted therapy. These results are consistent with other studies of real-world dataCitation15,Citation23–25 and also indicate that sequential use of VEGF inhibitors results in cumulative toxicities, a situation which may pose management challenges for patients and physicians in the community setting. The impact of cumulative toxicity warrants further investigation in future research on treatment sequencing.
At the time of our analysis, following a first-line VEGF inhibitor, physicians reported that they would choose an mTOR agent more often than a VEGF inhibitor in the second-line setting, regardless of prognosis. This finding was largely consistent with the observed treatment sequences recorded from the chart data, with small differences that may be due to recall bias or imprecision in selecting a preferred sequence for hypothetical patients characterized only by prognosis. The finding that mTOR agents are more often selected in the second-line setting is also consistent with previously presented results of a survey of medical oncologistsCitation26.
Unadjusted durations of PFS and OS after the initiation of second targeted therapies were similar for the treatment sequences VEGF–mTOR and VEGF–VEGF. These durations are longer than those observed in clinical trials for second-line mRCC treatment, potentially due to less stringent use of imaging and RECIST outside of clinical trials and a different case mix in the community setting.
The present study described real-world observations, and thus several caveats should be applied to its interpretation. First, as a descriptive study, it should be noted that comparisons between therapies were not adjusted for differences in patient characteristics and may not represent true effects of different drugs. For example, the patients using different drugs differed in baseline characteristics that could have impacted outcomes, including demographics (e.g. age, gender, and race), mRCC duration, MSKCC risk status, number and sites of metastasis, response and progression on the first targeted therapy, prior treatments received for mRCC, and comorbidities. Further assessment of the comparative effectiveness of various sequences of targeted agents used in mRCC treatment is beyond the scope of the current manuscript; this information is reported in a separate manuscript (with adjustments for patient characteristics as discussed above)Citation27. It should also be noted that physicians’ reporting of progression in the real-world setting could differ from central review in clinical trials. In this study progression was defined by the participating physician, based on worsening cancer-related symptoms, radiographic evidence or other evidence. Moreover, given the relatively short period of time since axitinib was available and small sample size, its treatment patterns could not be completely assessed. Lastly, some of the treatment sequences used in this real-world practice report are neither supported by prospective data (e.g., the use of bevacizumab in a third-line setting, after a TKI and an mTOR), nor feasible in many countries outside the US due to regulatory restrictions in those countries, thus limiting the transferability of these observations.
Conclusion
In this large, retrospective chart review, VEGF–mTOR–VEGF was the most commonly observed treatment sequence for mRCC in community oncology settings for the observed study period. Sunitinib followed by everolimus was the most commonly used treatment sequence for first- and second-line targeted therapies. Published efficacy data, treatment guidelines and mechanism of action were the main reasons for choice of therapy in both lines of treatment. Rates of discontinuation due to toxicity were higher for second-line VEGF inhibitor than for second-line mTOR inhibitors. Additional evidence from randomized controlled trials is needed to confirm optimal treatment sequences in mRCC.
Transparency
Declaration of funding
Research was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Author contributions: All authors participated in the design of the study and contributed to the manuscript development. J.E.S. and P.L.L. conducted analyses.
Declaration of financial/other relationships
E.J. has disclosed that he has received grants from and has been a consultant to GSK, Novartis and Pfizer. J.E.S. and P.L.L. have disclosed that they are employees of Analysis Group Inc., a company that received funding from Novartis Pharmaceuticals Corporation to conduct this study. Z.L. and K.C. have disclosed that they are employees of Novartis Pharmaceuticals Corporation. S.K.P. has disclosed that he has been a consultant for Novartis, Pfizer, Aveo, Denelneon, and Myriad and has spoken at Novartis, Pfizer and Medivation. N.J.V. has disclosed that he has been a consultant for Novartis, Amgen, Celgene, Medivation, Eisai, Exelixis, Roche, has spoken at Novartis, Astellas, Johnson and Johnson, Pfizer, Dendreon, Bayer/Algeta, GSK, and Veridex/Janssen, and has received research support from Novartis, Bayer, Exelixis, Progenics, Bavarian Nordic, and Viamet. J.A.S. has disclosed that he has no significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no relevant financial or other relationships to disclose.
Acknowledgements
A synopsis of the current research was presented in poster format at the 2013 Genitourinary Cancers Symposium, which took place in Orlando, FL, during February 14–16, 2013
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29
- Flanigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol 2003;4:385-90
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology – Kidney Cancer version 3.2014. Fort Washington, PA: National Comprehensive Cancer Network, 2014. Available at: http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf [Last accessed 14 May 2014]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol Off J Am Soc Clin Oncol 2009;27:3312-18
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol Off J Am Soc Clin Oncol 2010;28:2144-50
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol Off J Am Soc Clin Oncol 2010;28:1061-8
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34
- Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol 2014;32:760-7
- Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:286-96
- Busch J, Seidel C, Kempkensteffen C, et al. Sequence therapy in patients with metastatic renal cell carcinoma: comparison of common targeted treatment options following failure of receptor tyrosine kinase inhibitors. Eur Urol 2011;60:1163-70
- Chen C-C, Hess GP, Liu Z, et al. Second-line treatment outcomes after first-line sunitinib therapy in metastatic renal cell carcinoma. Clin Genitourin Cancer 2012;10:256-61
- Feinberg BA, Jolly P, Wang S-T, et al. Safety and treatment patterns of angiogenesis inhibitors in patients with metastatic renal cell carcinoma: evidence from US community oncology clinics. Med Oncol Northwood Lond Engl 2012;29:786-94
- Park K, Lee J-L, Park I, et al. Comparative efficacy of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI) and mammalian target of rapamycin (mTOR) inhibitor as second-line therapy in patients with metastatic renal cell carcinoma after the failure of first-line VEGF TKI. Med Oncol Northwood Lond Engl 2012;29:3291-7
- Vickers MM, Choueiri TK, Rogers M, et al. Clinical outcome in metastatic renal cell carcinoma patients after failure of initial vascular endothelial growth factor-targeted therapy. Urology 2010;76:430-4
- Giuliani J, Drudi F. Immunotherapy and targeted therapies in metastatic renal cell carcinoma: is there a preferred sequence? Cancer Biother Radiopharm 2012;27:513-18
- Larkin J, Swanton C, Pickering L. Optimizing treatment of metastatic renal cell carcinoma by changing mechanism of action. Expert Rev Anticancer Ther 2011;11:639-49
- Porta C, Sabbatini R, Procopio G, et al. Primary resistance to tyrosine kinase inhibitors in patients with advanced renal cell carcinoma: state-of-the-science. Expert Rev Anticancer Ther 2012;12:1571-7
- Wong MK, Yang H, Signorovitch JE, et al. Comparative outcomes of everolimus, temsirolimus and sorafenib as second targeted therapies for metastatic renal cell carcinoma: a US medical record review. Curr Med Res Opin 2014;30:537-45
- Di Lorenzo G, Casciano R, Malangone E, et al. An adjusted indirect comparison of everolimus and sorafenib therapy in sunitinib-refractory metastatic renal cell carcinoma patients using repeated matched samples. Expert Opin Pharmacother 2011;12:1491-7
- Heng D. A population-based overview of sequences of targeted therapy in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2012;30(Suppl 5):abstr 387
- Yang H, Wong MKK, Signorovitch JE, et al. Overall and progression-free survival with everolimus, temsirolimus, or sorafenib as second targeted therapies for metastatic renal cell carcinoma: a retrospective U.S. chart review. J Clin Oncol 2012;30:(suppl): abstr 4612
- Ryan CJ, Green MR, Britton SL, et al. Second-line prescribing preferences (PPrefs) of U.S.-based medical oncologists (MOs) following first-line TKI in patients with metastatic clear cell renal cell carcinoma (mccRCC). J Clin Oncol 2014;32(suppl 4): abstr 492
- Signorovitch JE, Vogelzang NJ, Pal SK, et al. Comparative effectiveness of second-line targeted therapies for metastatic renal cell carcinoma: synthesis of findings from two multi-practice chart reviews in the United States. Curr Med Res Opin 2014, in press

