Abstract
Backgrounds and objectives:
Increasing use of oral anticancer treatments (OATs) in oncology is modifying the treatment paradigm for cancer. Nonetheless, available data on the pattern of use of OATs and its evolution over time are limited. The objective of this study was to describe the patterns of use of OATs in France from 2004 to 2012.
Methods:
A retrospective analysis was performed using Oncology Analyzer, a physician survey database. All patients actively treated by an oral or an intravenous anticancer treatment between October 2004 and September 2012 were enrolled in the database. Descriptive analyses were performed by treatment category with a focus on the last year of collection and the evolution across the study period.
Results:
From October 2011 to September 2012, a sample of 7426 patients treated by oral or intravenous active anticancer treatments was analyzed: 74% of patients receiving an OAT were diagnosed with a solid tumor, 52% of whom had a stage IV cancer. The use of OATs increased with age and was the highest in patients over 80 years. From 2004 to 2012, the proportion of cancer patients receiving OATs increased by four percentage points (from 28.4% to 32.5%). Additionally, for treatments available in both forms, a marked preference for oral formulations was observed.
Limitations:
The patterns and trend of use prior to 2004 were not addressed due to lack of information in the database. The use of a market research database is relevant for highly prevalent cancers but for rare cancers the sample size is limited, underlining the utility of using other data sources such as cancer registries.
Conclusions:
The Re-ACTOR study provides an overview of OAT use in France, which was prescribed to 32% of cancer patients in France in 2012, principally to older patients and to those with solid tumors and with metastatic disease.
Introduction
Historically, anticancer treatments have generally been administered by the intravenous (IV) route during an outpatient visit. However, the recent introduction of oral anticancer treatments (OATs) has led to a shift in the management of patients suffering from cancer from outpatient settings to home-administered treatmentsCitation1. This trend is expected to continue, since it was estimated that in 2008, 25%–30% of molecules under development in oncology were designed for oral administration and 40% of OATs have been approved within the last 7 yearsCitation1,Citation2. In addition, the majority of patients suffering from advanced cancers prefer OATs to IV treatments as long as similar efficacy can be guaranteedCitation3. A Canadian study conducted in 135 cancer patients on palliative care revealed that 89% of these patients preferred to be treated with an oral regimen if efficacy was not compromisedCitation4. The oral route is perceived as more convenient than the IV route because it reduces the impact of treatment on daily life activities and avoids outpatient clinical visits for infusionsCitation5,Citation6.
Nevertheless, serious concerns have been raised relating to the use of OATs and the change in cancer treatment paradigmCitation1. In particular, this novel approach to drug delivery shifts the responsibility of treatment administration from healthcare providers to the patientCitation1. Traditionally, cancer treatment was administered by an expert oncologist during an outpatient hospitalization where treatment was supervised and patients were regularly monitored. In contrast, with the use of medications self-administered by the patient at home, patients may make fewer visits to the oncologist, which makes it more difficult for physicians to monitor adherence to treatmentsCitation2. The safety profile of OATs may differ from their IV counterparts and adverse events usually occur at home in the absence of healthcare professionals to monitor and manage these side effectsCitation2. Furthermore, forgetfulness as well as safety or tolerability issues may lead to a suboptimal adherence to treatment. Indeed, a study has shown a significant correlation between the number of adverse events and adherence to OATsCitation7.
Even though it is believed that cancer patients generally adhere well to treatment regimens, studies have shown that adherence to OATs and persistence are a major concern, especially since they may affect treatment efficacyCitation2,Citation8. In addition, non-adherence and non-persistence are associated with an elevated rate of healthcare resource utilization and consequently an increased economic impactCitation8.
Finally, new oral anticancer treatments are often expensive and may have significant impact on National Health Insurance (NHI) cost outlay. For example, the total cost of oral anticancer treatments covered by National Health Insurance in France in 2011 was estimated to be €623,175,583, representing 4% of the total cost for drug reimbursement in France. In contrast, the expenses related to IV anticancer treatments, which are often relatively old drugs available as generics or at a lower cost, account for only 1% of the total cost for drug reimbursementCitation9. However, when the oral treatment replaces an IV administered molecule, the increased costs of the OAT might be balanced by savings due to reduced clinical visits for infusion and avoiding expenses related to disposals such as infusion equipmentsCitation4,Citation10.
In spite of soaring use of OATs over the past decade and the concerns surrounding the change in cancer treatment paradigm, there are to our knowledge limited data available describing the patterns of use of oral anticancer agents. For this reason, the present study was undertaken in order to describe the patterns of use and the evolution of oral anticancer agents in France from October 2004 to September 2012.
Materials and methods
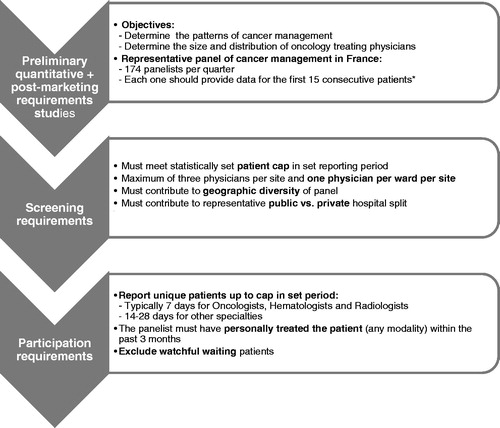
A retrospective analysis was performed using Oncology Analyzer (IMS Health, France), a physician survey available since 2004 in Europe (France without overseas territories, Germany, Italy, Spain, Netherlands and the United Kingdom) and Asia (China, Korea, Japan, Taiwan and Turkey). The database documents patient care in oncology with respect to diagnosis and treatment patterns (current and past) and demographic characteristics. Cancer patients are identified using the International Classification of Diseases codes version 10 (ICD-10) for malignant neoplasms, neoplasms of uncertain or unknown behavior and in situ neoplasms. The sampling plan is set up in order to be representative of physician specialties treating cancer in each country. The sample size is based on a preliminary quantitative study conducted by IMS with more than 3000 specialists in seven countries in order to determine the patterns of cancer management (physicians treating each cancer type and the number of patients treated by each physician). The number and distribution of hospital-based physicians treating cancer was estimated separately through IMS primary market research studies. On this basis, it was estimated that 174 specialists in France (radiotherapists, dermatologists, otolaryngologists, gastroenterologists, general surgeons, gynecologists, hematologists, oncologists, pulmonologists and urologists) working in different French regions (urban and rural areas) and in different treatment settings (public hospitals and private clinics) should be included in the Oncology Analyzer panel in order to capture a representative picture of cancer care in France. To obtain a representative sample of cancer patients, panelists were expected to provide data for the first 15 consecutive patients per quarter consulting during each recruitment period (). Enrolled patients had to be actively treated (no watchful waiting). Anonymous medical information was retrospectively collected through paper or web-based questionnaires completed by the panelists from patient medical records, collecting historical information from diagnosis until the current treatment at the time of data collection. In this database, patients were included on a quarterly basis and were not followed longitudinally over time. In order to avoid duplicates, each panelist was required to declare that the patient was not previously reported in the study.
Figure 1. Summary of the Oncology Analyzer methodology. *Participation in the panel was restricted to physicians who could be expected to include the target number of 15 patients for each quarter.
In France, the survey covers more than 10000 cancer patients per year. In 2012, 130 French institutions participated in the survey, namely 88 public hospitals, 16 private institutions and 26 private non-profit institutions.
For this study, data were collected between October 2004 and September 2012 for all patients diagnosed with cancer, regardless of disease stage, and treated by OATs or IV anticancer treatments or both. Intravenous routes of administration included intravenous bolus, infusion and continuous infusion. Patients treated by other routes of administration such as intramuscular administration, were excluded from the analysis, as were those treated with other therapeutic approaches, such as radiotherapy or surgery.
The Anatomical Therapeutic Chemical (ATC) Classification System from the European Pharmaceutical Marketing Research Association (EphMRA) was used to define different treatment categories (). Due to the large number of supportive care drugs that are orally administered (ATC codes: B3C, L3A1, A4A, N1A2, N2A, N2B, M5B4 and M5B9), patients receiving exclusively this treatment category were also excluded from the analyses, in order to avoid bias in the estimation of the proportion of OATs.
Table 1. Treatment categories used in the analyses according to EphMRA classification.
Finally, eligible patients were divided into six categories according to the type of therapy administered. These were (1) standard chemotherapy including all patients treated by standard cytotoxic drugs (ATC codes: L1A, L1B, L1C, L1D, L1F), (2) other antineoplastic agents including patients treated by novel cytotoxic agents (ATC code L1X9), (3) targeted therapy, including all patients treated by monoclonal antibodies or tyrosine-kinase inhibitors (ATC codes: L1G, L1H), (4) hormonal therapy (ATC code L2), (5) immunostimulants (ATC code L3) and (6) immunosuppressants (ATC code L4). It should be noted that lenalidomide and thalidomide are antiangiogenic agents and not, strictly speaking, immunotherapies, although they are categorized as immunosuppressants in the EphMRA ATC classification.
Statistical analysis
Statistical analysis was conducted using Microsoft Office Excel 2007 software.
Given the objectives, the analyses were principally descriptive. Continuous variables were described by the number of valid cases, mean values and standard deviation. Regarding age, only the age range was available in the database. For this reason, the estimated average is a proxy one based on age distribution in 5 year intervals: the median value of the range was considered for each interval, except for the extreme intervals (0–1 years age class, for which the value 1 was used, the >80 years class, for which the value 85 was used). Categorical variables were described as the total number and relative percentage per category. Missing data were not replaced by imputation, as they were infrequent and assumed to be distributed at random. Analyses were performed on the unadjusted data. No projection factor was applied to generalize the results to the entire cancer population.
The results are presented by route of administration and type of administered therapy and as the number of treated patients and the molecules prescribed. Patterns of use of OATs were also analyzed according to cancer stage; this analysis was performed exclusively on patients diagnosed with solid tumors since the Tumour, Nodes, Metastasis (TNM) staging system is not used to assess liquid tumors.
Detailed description of patients and treatments was performed for the cohort of patients included from the fourth quarter of 2011 (4Q 2011) to the third quarter of 2012 (3Q 2012). In addition, trends in the aggregate data between 2004 and 2012 were assessed from a moving annual aggregate for each year X covering the period from the fourth quarter of Year X - 1 to the third quarter of Year X.
Results
Patient characteristics
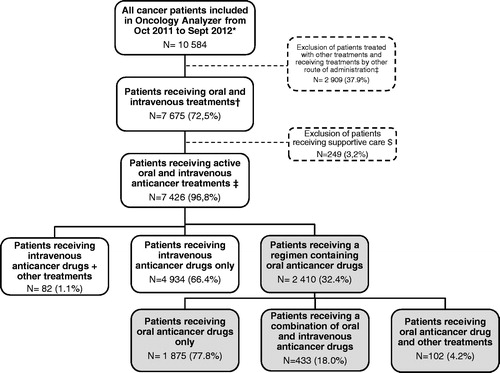
A sample of 7675 cancer patients included in Oncology Analyzer between 4Q 2011 to 3Q 2012, treated by at least one oral or IV treatment including supportive care drugs, and potentially eligible for the study was identified. Of these, 7426 patients (97%) were treated with OATs or IV treatments and were included in the analyses (). The male/female ratio was 47%/53% and the median age was 63 years. Patients were mostly treated for breast cancer (24%), non-small-cell lung cancer (NSCLC) (13%), colorectal cancer (11%), non-Hodgkin lymphoma (NHL) (6%), head and neck cancer (5%) and pancreatic cancer (5%). Age and gender distribution were stable over the study period. However, the cancer type distribution varied across the study period, especially for patients with NSCLC (9.8% in 2005 vs. 12.8% in 2012), head and neck cancer (2.5% vs. 5.5%), kidney cancer (0.6% vs. 2.8%), pancreatic cancer (3.1% vs. 4.6%), NHL (10.9% vs. 6.1%), all others (i.e. rare cancers) (5.3% vs. 3.6%) and prostate cancer (3.4% vs. 1.8%).
Figure 2. Flow chart of patients included in the analyses between October 2011 and September 2012.*Figures presented are for those included in Oncology Analyzer between October 2011 (4Q2011) and September 2012 (3Q2012). †Intravenous treatments were defined as intravenous, bolus, infusion or continuous infusion. ‡Patients treated with other treatments (i.e. radiotherapy and surgery) and treated with chemotherapy by other route of administration (subcutaneous, intravesical…) were excluded from the analysis. $ATC codes B3C, L3A1, A4A, N1A2, N2A, N2B, M5B4 and M5B9 were excluded from the analysis.
Treatment categories
In 2012, 119 distinct oral and IV anticancer treatments were prescribed in France, of which 56 (47%) were available exclusively in IV formulations, 43 (36%) exclusively in oral formulations, 11 (9%) in both IV and oral formulations and 9 (8%) available in IV formulations and formulations other than oral. Overall, 54 molecules were available in an oral formulation (45%). Individual OATs were represented in standard chemotherapy (20 OATs; 31% of all standard chemotherapy), targeted therapies (15 OATs; 58%), hormonal therapy (12 OATs; 67%), immunosuppressants (3 OATs; 75%) and other antineoplastic agents (4 OATs; 29%).
In 2005, 91 different oral and IV anticancer treatments were prescribed in France, of which 41 (45%) were available in oral formulations. Between 2005 and 2012, the relative weight of oral formulations among the available active anticancer treatments has remained constant, even if thirteen additional molecules have been developed as oral formulations during the intervening period. With respect to drug categories, the highest growth was observed for targeted therapies, where the number of molecules available in oral formulations increased from four to fifteen.
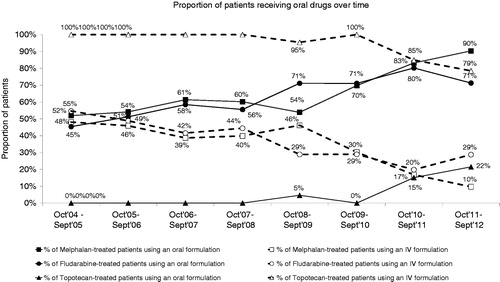
For molecules which were available in both IV and oral formulations, such as melphalan, methotrexate, etoposide and vinorelbine, there was a progressive shift in favor of oral formulations between 2005 and 2012 ().
OATs use and patient characteristics
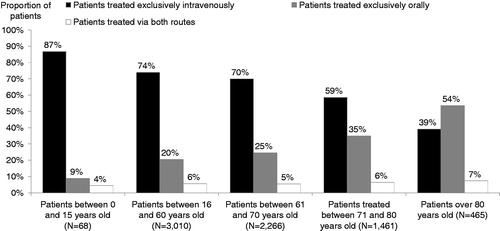
In 2012, 32% (N = 2410/7426) of the eligible population received a regimen containing at least one oral anticancer treatment. Among these, 78% received oral regimens exclusively, 18% received a combination of oral and IV treatments and 4% received a combination of treatments administered orally and treatments administered by another route. A diagnosis of a solid tumor was documented in 74% of patients receiving OATs. The six most frequent cancer types for which OATs were prescribed were breast cancer (34%), multiple myeloma (8%), NSCLC (8%), chronic myeloid leukaemia (CML) (8%), colorectal cancer (7%) and kidney cancer (7%). Use of OATs exclusively increased with age, accounting for 54% of patients over 80 years old compared to 20% of those aged between 16 and 60 years (). This descriptive result was not controlled by tumor type.
Figure 4. Age distribution and route of treatment administration for patients included between October 2011 and September 2012.
From 2005 to 2012, the number of cancer patients receiving OATs across all treatment categories increased by four percentage points from 2241/7891 (28.4%) to 2410/7426 (32.5%). The use OATs across the study period mainly increased in elderly patients. The proportion of patients aged between 61 and 70 years using OATs increased from 26% in 2005 to 30% in 2012, and the proportion of patients aged >70 years increased from 40% to 46% across the same period. In addition, the proportion of patients diagnosed with a solid tumor who were prescribed an OAT increased by six percentage points from 1532/2241(68%) to 1787/2410 (74%). With respect to cancer stage, use of OATs increased in patients with metastatic cancer (from 37% to 52%) and decreased in patients with stage II cancer ().
Table 2. Distribution of patients by year according to the route of administration, the treatment category and cancer stage.
Overall, the use of OATs was highest in patients aged more than 71 years old. Additionally, the analyses showed that OATs were mainly prescribed in patients diagnosed with a solid tumor and more specifically in those suffering from a metastatic cancer (stage IV).
Patterns of OATs use by treatment category
Targeted therapy
The population treated with targeted therapies increased over time across both forms (IV or oral) (1084 in 2005 vs. 2225 in 2012).The study showed that among patients treated with targeted therapies 33% in 2012 received an oral regimen compared to 22% in 2005. Furthermore, oral targeted therapies covered more than 80% of the overall targeted therapies indications. Of patients receiving an orally administered targeted therapy 59% were men. Of patients treated by oral targeted therapies 73% were diagnosed with a solid tumor, 86% of whom had a stage IV cancer. Patients receiving oral targeted therapies were mainly diagnosed with CML (25%), kidney cancer (23%), NSCLC (23%) and liver cancer (14%).
Standard chemotherapy
Standard chemotherapies were mainly administered by IV route; only 14% of patients were treated orally, this proportion remained stable across the study period. Oral treatments covered nearly 75% of cancers treated with standard chemotherapies. These treatments were mainly prescribed in colorectal cancer (22%), breast cancer (13%), brain cancer (13%), chronic lymphoid leukemia (CLL) (13%) and multiple myeloma and plasma cell (12%). 52% of patients diagnosed with a solid tumor and treated by oral standard chemotherapies had a stage IV cancer.
Other antineoplastic agents
Unlike the other categories, there was a decrease from 51% to 16% in the use of second generation chemotherapies delivered by oral route of administration between 2005 and 2012. This class of treatment was mainly used to treat patients diagnosed with myelodysplastic syndromes.
Other treatment categories
The analysis showed that 99% of patients receiving hormonal therapy in 2012 were treated with OATs. These patients were mainly women (sex ratio male/female 11%/89%). Furthermore, of the 18 molecules available in this treatment category in 2012, 67% were orally administered. This type of molecule was mainly prescribed in patients diagnosed with hormone-sensitive cancers such as breast cancer (87%) and prostate cancer (11%). The use of oral hormonal therapy and oral immunotherapy was stable across the study period. Finally, there were no orally administered drugs in the immunostimulant treatment category.
Oral treatments used per type of molecule
In 2012, across all treatment categories (N = 2410 patients), the most prescribed molecules were anastrozole (12%), capecitabine (12%), letrozole (11%) and imatinib (6%) (). Among oral standard chemotherapies (N = 753 patients), the most prescribed molecules were capecitabine (38%), temozolomide (13%), cyclophosphamide (12%) and melphalan (11%). Among oral targeted therapies (N = 729 patients), the treatments most prescribed were imatinib (20%), sunitinib (18%), sorafenib (16%), erlotinib (14%) and gefitinib (12%). Regarding the other treatment categories, hydroxycarbamide represented 88% of all oral second generation chemotherapies (N = 42 patients). Anastrozole, letrozole and tamoxifen accounted for 35%, 31% and 16% respectively of all oral hormonal therapy (N = 837 patients); lenalidomide and thalidomide accounted for approximately 99% of all oral immunosupressants (N = 125 patients).
Table 3. Distribution of patients receiving OATs by molecule between October 2011 and September 2012 (N = 2410).
Discussion
In recent years, a shift of cancer treatment from the hospital to the home has occurred as a result of increasing use of oral anticancer treatments (OATs). This study describes cancer treatment patterns with respect to the use of OATs in France from 2004 to 2012 for all types of cancer. The panel design ensures that all regions and all types of health institutions are covered, so it provides a reliable picture of cancer management in France. The methodology of data collection also ensures that all types of patients across all treatment modalities and all major cancer types are covered. However, since this study included exclusively patients who were actively treated by an oral or an IV anticancer treatment, the structure of the included sample in terms of cancer type is highly dependent on the availability of oral or IV anticancer treatments for each cancer type and their use. This methodology limits the comparison of the characteristics of the included sample with existing data in the literature. Until now, the extent of use of anticancer treatments available in oral formulations is poorly characterized and may vary across countries. Published data from the literature provides widely varying estimates of the extent of OAT use, ranging from 10% to 25%Citation2,Citation11. Our retrospective database analysis, conducted on a sample of 7426 patients and 119 distinct oral and IV anticancer treatments in 2012, showed that 45% of anticancer treatments were available in oral form in France. Comparisons with other published data should be interpreted with caution since the number of oral anticancer treatments may vary from one country to another depending on marketing authorizations.
Our findings show that the proportion of anticancer drugs that were available in oral formulations was relatively stable over the period 2005–2012, despite a fourfold increase in the number of oral targeted therapies available (from 4 to 15). However, the proportion of patients treated with OATs increased slightly. In 2012, 32% of the study population were treated with at least one OAT. This proportion increased with age, perhaps reflecting a preference for the oral route of administration among the elderly, related to preserved quality of life due to the non-invasive route of administration, fewer outpatient hospitalizations and lower impact on daily life. However, the preferential use of OATs in the elderly population raises potential concerns with respect to problems swallowing, treatment adherence, drug interactions due to polymedication and lack of family support in disease management. We also showed that nearly three-quarters of patients receiving OATs were treated for a solid tumor, which might be explained primarily by the larger number of treatments available for this type of tumor compared to those available for hematological malignancies, and by the higher prevalence of solid tumors. Moreover, in patients treated for a solid tumor, the use of OATs was the highest in those with stage IV metastatic cancer. This could be explained by the fact that most OATs are specifically indicated for the treatment of metastatic cancer.
A shift in the types of cancer included over time was observed. These findings were consistent with the availability and the use of new treatments for each cancer type and the evolution of the incidence of cancer across the study periodCitation12,Citation13. Given the availability of new treatments for some cancer types, these findings might not be valid for another time period. Among treatments available in both IV and oral formulations, such as melphalan, fludarabine and topotecan, a shift towards use of the oral formulation at the expense of the IV formulation was observed since 2009. This observation is consistent with previous studies demonstrating patient preference for the oral route of administrationCitation4,Citation14. The database used for this study is available in other European countries and it would be interesting to compare the patterns of use of OATs between countries and assess any impact of the organization or funding of healthcare provision on OAT use, bearing in mind potential differences in reimbursement criteria or marketing authorization between countries.
This study has several limitations. Firstly, no information is available in the database prior to 2004, so the study could only address the evolution of the use of OATs since that year even though the first OATs were available in the late 1990s. The possibility cannot be ruled out that patterns of OAT use were different in the earlier period.
Secondly, as participating physicians were asked to include only the first 15 consecutive patients seen during each quarter, severe or frequent cases of cancer who consult more frequently might be over-represented in the database and, in contrast, patients with rare cancers might be under-represented. In consequence, the distribution of cancer types observed in our sample may not necessarily exactly match the epidemiology of cancers in FranceCitation15. Although the source database is designed to be representative of cancer care in each participating country, it is also possible that the distribution of treatment settings may not correspond exactly to the pattern of cancer care provision in France. Nonetheless, treatment patterns are not expected to vary markedly between treatment settings and such a potential mismatch would not thus be expected to have an important impact on the findings.
Thirdly, this study provides descriptive information on patterns and trends of use of OATs, but cannot address the reasons underlying the choice of OAT treatments. Further research, for example using multivariate analysis, would be useful to understand why and how particular OATs are used. We initially planned to take advantage of the extensive retrospective information contained in this database relating to cancer type, patient profile, healthcare organization and the reimbursement system. However, the multivariate analysis was not conducted due to a lack of relevant information in the database and a limited sample size per cancer type. Indeed, for a given efficacy/safety profile, many factors such as economic considerations, patients characteristics (socio-demographic factors, perception of social support, patient’s preference, factors affecting adherence like psychological status, etc.) can influence the decision to prescribe oral versus IV anticancer treatments. Most of these data are not collected in the databaseCitation16–19. Additionally, to provide robust results and to avoid over-parameterization, a large sample size is required. But since the distribution of cancer types within the included sample depends on the prevalence of each cancer type and the use of oral and IV treatments, a sufficient number of patients was available only for the most frequent cancer types. Therefore, to perform multivariate analysis, it might be useful to use better adapted data sources such as cancer registries.
Conclusions
The Re-ACTOR study was designed to provide a first description of the patterns of use of OATs in France and to provide a better understanding of cancer patients treated with OATs. Further research such as multivariate analyses would be useful to explore the determinants of the prescription of an oral anticancer treatment. The proportion of cancer patients treated with OATs increased between 2004 and 2012 in France, mainly in elderly people and for the metastatic stage of cancer. This finding highlights the need to reinforce accompanying measures for OAT prescription in terms of safety and healthcare delivery, especially for the elderly who require specific attention to provide them with adequate patient support tools and programs.
Transparency
Declaration of funding
This study was funded by GlaxoSmithKline.
Declaration of financial/other relationships
L.B. and R.M. have disclosed that they are employees of GlaxoSmithKline, a purveyor of several oral anticancer treatments. F.M., I.B. and C.R. have disclosed that they are employees of IMS Health, a consultancy firm that received funding from GlaxoSmithKline to perform the analyses reported in this manuscript. All authors had full access to the data and had final responsibility for the decision to submit the manuscript.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Adam Doble (Foxymed, Paris, France) for stylistic assistance with the manuscript.
Previous presentation: Results were partially presented at the 16th European ISPOR congress, 2–6 November 2013, Dublin (PCN 188, PCN 185).
References
- Shen C, Chien CR, Geynisman DM, et al. A review of economic impact of targeted oral anticancer medications. Expert Rev Pharmacoeconomics Outcomes Res 2014;14:45-69
- Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw 2008;6(Suppl 3):S1-14
- Borner M, Scheithauer W, Twelves C, et al. Answering patients' needs: oral alternatives to intravenous therapy. Oncologist 2001;6(Suppl 4):12-16
- Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 1997;15:110-15
- Colomer R, Alba E, Gonzalez-Martin A, et al. Treatment of cancer with oral drugs: a position statement by the Spanish Society of Medical Oncology (SEOM). Ann Oncol 2010;21:195-8
- Wood L. A review on adherence management in patients on oral cancer therapies. Eur J Oncol Nurs 2012;16:432-8
- Geynisman DM, Wickersham KE. Adherence to targeted oral anticancer medications. Discovery Med 2013;15:231-41
- Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA: A Cancer Journal for Clinicians 2009;59:56-66
- Situation de la chimiothérapie des cancers. Institut National Du Cancer 2012. Available at: http://www.e-cancer.fr/en/rss-soins/7763-rapport-annuel-sur-la-situation-de-la-chimiotherapie-des-cancers [Last accessed 25 March 2014]
- O’Neill VJ, Twelves CJ. Oral cancer treatment: developments in chemotherapy and beyond. Br J Cancer 2002;87:933-7
- Halfdanarson TR, Jatoi A. Oral cancer chemotherapy: the critical interplay between patient education and patient safety. Curr Oncol Rep 2010;12:247-52
- Estimation nationale de l’incidence et de la mortalité par cancer en France entre 1980 et 2012: Etude à partir des registres des cancers du réseau Francim – Partie 1: tumeurs solides. Institut National de Veille Sanitaire, 2013. Available at: http://www.invs.sante.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-chroniques-et-traumatismes/2013/Estimation-nationale-de-l-incidence-et-de-la-mortalite-par-cancer-en-France-entre-1980-et-2012 [Last accessed 27 October 2014]
- Estimation nationale de l’incidence des cancers en France entre 1980 et 2012: Étude à partir des registres des cancers du réseau Francim Partie 2 – Hémopathies malignes. Institut National de Veille Sanitaire, 2013. Available at: http://www.invs.sante.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-chroniques-et-traumatismes/2013/Estimation-nationale-de-l-incidence-des-cancers-en-France-entre-1980-et-2012 [Last accessed 27 October 2014]
- Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Canc Res Treat 2005;92:265-72
- Les cancers en France. Institut National Du Cancer, 2013. Available at: http://www.e-cancer.fr/publications/69-epidemiologie/758-les-cancers-en-france-edition-2013 [Last accessed 17 February 2014]
- Benjamin L, Buthion V, Vidal-Trécan G, Briot P. Impact of the healthcare payment system on patient access to oral anticancer drugs: an illustration from the French and United States contexts. BMC Health Serv Res. 2014;14:274
- Van Nuys K, Conoshenti J, Goldman D. Practice economics and the decision to prescribe oral oncolytics. Am J Pharm Benefits 2013;5(Special Issue):SP29-31
- Pertinence du développement de la chimiothérapie en Hospitalisation à domicile: analyse économique et organisationnelle. Haute Autorité de Santé, 2013. Available at: http://www.has-sante.fr/portail/jcms/c_1696038/fr/pertinence-du-developpement-de-la-chimiotherapie-en-hospitalisation-a-domicile-analyse-economique-et-organisationnelle [Last accessed 27 October 2014]
- Lee YH, Jeong IS. Factors influencing medication adherence to oral anticancer drugs [Korean]. Asian Oncol Nurs 2013;13:201-9