Abstract
Background:
Infections caused by multi-drug-resistant Gram-negative bacteria, particularly Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae, that cause nosocomial infections, represent a growing problem worldwide. The rapid increase in the prevalence of Gram-negative pathogens that are resistant to fluoroquinolones and aminoglycosides as well as all β-lactams, including carbapenems, monobactam, cephalosporins and broad-spectrum penicillins, has prompted the reconsideration of colistin as a valid therapeutic option. Colistin is an old class of cationic, which act by disrupting the bacterial membranes resulting in cellular death. Although there has been a significant recent increase in the data gathered on colistin, focusing on its chemistry, antibacterial activity, mechanism of action and resistance, pharmacokinetics, pharmacodynamics and new clinical application, the prevalence of colistin resistance has been very little reported in the literature. This review concentrates on recent literature aimed at optimizing the clinical use of this important antibiotic.
Methods:
The available evidence from various studies (microbiological and clinical studies, retrieved from the PubMed, and Scopus databases) regarding the mechanisms and prevalence of resistance was evaluated.
Results:
Increasing use of colistin for treatment of infections caused by these bacteria has led to the emergence of colistin resistance in several countries worldwide. Although resistance to polymyxins is generally less than 10%, it is higher in the Mediterranean and South-East Asia (Korea and Singapore), where colistin resistance rates are continually increasing.
Conclusion:
There is a critical need for effective infection prevention and control measures and strict use of antibiotics in the world to control the rise and spread of colistin resistance.
Introduction
The world is facing an enormous and growing threat from the emergence of Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae. Development of resistance to most available antibiotics including β-lactams, carbapenems, fluoroquinolones, and aminoglycosides is increasing worldwide at an alarming rate. Therefore the lack of development of new antimicrobial agents has prompted the medical community to re-evaluate the use of colistin in many health care centers around the world, where no other less toxic or effective antibiotic is availableCitation1–3. In the past years, interest has been rekindled in the ‘old’ polymyxin antibiotic, colistin (polymyxin E), for the treatment of infections caused by multi-drug-resistant Gram-negative bacteria (MDR-GNB) owing to its favorable properties of rapid bacterial killing, a narrow spectrum of activity, and an associated slow development of resistanceCitation4. However, these salvage antibiotics were not available in the market in some countries for the treatment of patients with infections due to (inappropriately called) pan-drug-resistant Gram-negative bacteria, since isolates were susceptible to polymyxinsCitation5.
Most studies report that resistance to carbapenems is increasing and, since tigecycline has not been registered in many countries, colistin is often the only effective antibiotic against multi-drug-resistant organismsCitation4. However, it is most notable for colistin activity toward MDR organisms including A. baumanni, P.aeruginosa and K. pneumonia, and current low levels of resistance have undergone as revival agents for treatment of infections caused by Gram-negative organisms.Citation1,Citation6,Citation7.
As described in the ‘Bad Bugs, No Drugs’ paper published by the Infectious Diseases Society of America (IDSA), “as antibiotic discovery stagnates, a public health crisis brews”Citation8. Therefore, there is an urgent need for new antibiotics, particularly those active against Gram-negative ‘superbugs’. This study research was done in order to optimizing the clinical use of this important antibiotic. To achieve this goal, almost all articles about microbiological and clinical studies in PubMed and Scopus databases regarding the mechanisms and prevalence of resistance were evaluated.
Early clinical experiences of colistin
One of the few remaining options left for treatment of life-threatening infections caused by MDR bacteria is colistin (or polymyxin E). It was first isolated in Japan by Koyama and co-workers from the spore-forming soil bacterium Bacillus polymyxa subsp. colistinus in 1947, and first used as an intravenous formulation in the 1950sCitation9–11. Colistin has been approved by the US FDA and has been available since 1959 for the treatment of infections caused by Gram-negative bacteria, because these agents are rapidly bactericidal against themCitation12,Citation13. During the ensuing decades, colistin was used in the treatment of several types of infections, including infectious diarrhea and urinary tract infections, as well as in bowel decontamination. Furthermore, polymyxins have been used for several decades in topical formulations for eye and ear infections and in regimens for selective bowel decontaminationCitation14. However, the high rates of toxicity associated with the use of polymyxins prompted their replacement by newer antibiotics like gentamicin and carbenicillin, two safer and more effective agents. Since the mid-1990s, there has been a greatly renewed interest in their clinical use due to prevalent MDR Gram-negative bacteria (especially P. aeruginosa, A. baumannii and K. pneumoniae) and lack of novel antibioticsCitation8. Recently, they were relegated to the status of a reserve agent after early reports of a ‘high’ incidence of toxicity. When the use of a β-lactam, aminoglycoside, or quinolone is ineffective, the polymyxins, particularly colistin, remain drugs of last resort for the treatment of infections with MDR Gram-negative superbugsCitation11.
Diversity and chemistry of colistin
Colistin, a polypeptide antibiotic of the polymyxin family, non-ribosomally synthesized with 1750 Da molecular weight, consisting of a cyclic heptapeptide with a tripeptide side chain acylated at the N terminus by a fatty acid tail (). It is noteworthy that the hydrophobicity of the N-terminal fatty acyl segment is responsible for the inherent toxicity and greatly influences the antimicrobial activity of colistinCitation15.
Figure 1. Colistin structures. (A) Structures of colistin A and B. (B) Structures of colistimethate A and B. Fatty acid: 6-methyloctanoic acid for colistin A and 6-methylheptanoic acid for colistin B; Thr = threonine; Leu = leucine; Dab = α,γ-diaminobutyric acid; α and γ indicate the respective amino groups involved in the peptide linkage. (Modified from Li et al.Citation4).
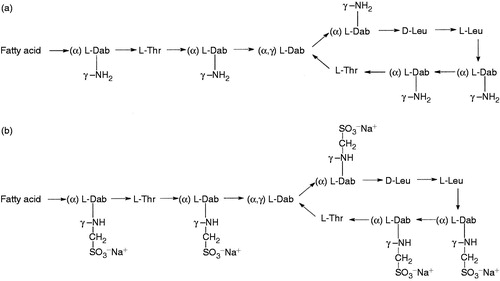
Polymyxin includes five different chemical compounds (polymyxins A, B, C, D, and E). It is mainly two of them, colistin A (polymyxin E1) and colistin B (polymyxin E2), that have been used in clinical practice; these differ only in their fatty acid tailsCitation1,Citation16. The difference between polymyxin B and colistin lies in the amino-acid componentsCitation17 and both are polycations at physiological pH owing to the five L-α,γ-diaminobutyric acid (Dab) residuesCitation18. There are two different forms of colistin available commercially, colistin sulfate for oral and topical use and colistimethate sodium (CMS) for parenteral and aerosol therapy; both forms may be given by inhalation. Colistin (usually used as the sulfate salt) is a polycation, whereas colistimethate (used as the sodium salt) is a polyanion at physiological pHCitation19.
CMS is an inactive prodrug of colistin and has no intrinsic antibacterial activity. CMS produced by a sulfomethylation reaction in which the primary amine groups on α,γ-diaminobutyric acid (Dab) are reacted with formaldehyde followed by sodium bisulfiteCitation16,Citation20. The composition must be converted to colistin in vivo, but this occurs slowly and incompletelyCitation3. CMS is increasingly the last line of defense for multi-drug-resistant Gram-negative bacteria and is now being used as ‘salvage’ therapy in critically ill patientsCitation21. CMS is less toxic than colistin when it is administered parenterallyCitation22,Citation23. As opposed to CMS which is rapidly hydrolyzed in plasma, colistin is stable for several days at room temperatureCitation24. There are several commercial preparations of colistimethate, and their differences have undoubtedly contributed to confusion when evaluating dosing guidelines. ‘Coly-Mycin M’ Parenteral is produced by Parkedale Pharmaceuticals in the United States. Another preparation of colistimethate is ‘Colomycin Injection’, manufactured by Alpharma ApS (Denmark)Citation25. Topical formulations of colistin sulfate and polymyxin B sulfate are also available in many countries. CMS is much more commonly used internationally (e.g. North America, South America, Asia, Europe and Australia) whereas parenteral polymyxin B is mainly available in the USA, Brazil and SingaporeCitation18. Nowadays, novel derivatives of polymyxins have been developed. Some of them, including NAB739, are directly antibacterial whereas others, including NAB7061, lack the direct activity but sensitize bacteria to other antibioticsCitation26.
Pharmacokinetics/pharmacodynamics
There is a dearth of reliable information concerning the pharmacokinetic (PK) and pharmacodynamic (PD) data available to guide dosing in humans especially critically ill patients, including those on renal replacement therapyCitation27. Most knowledge on the pharmacokinetics of colistin was obtained at least two decades ago when non-specific microbiological assays were used to measure the concentrations of colistin in biological fluidsCitation11. A better understanding of the PK/PD relationship of colistin is urgently needed to determine the optimal dosing regimen. Specifically, colistin concentrations in plasma of patients with preserved renal function have often been reported to be lower than expected and would be unlikely to be effective but may encourage resistanceCitation24.
The differences in chemistry between colistimethate and colistin also translate into differences in PK and PDCitation19. Colistin is a concentration-dependent antibiotic and if in the fermentation process the production of the subcomponent peptides varies in amount and composition, it could conceivably have an impact on antibacterial activity or on the synergistic effects that are apparent with complex antibiotics that contain mixed active antibiotic substances ()Citation15.
Figure 2. Pharmacokinetic (PK) simulation of colistin A and B in the in vitro PK model study (mean ± SD, n = 3). (From Zhao et al.Citation151).
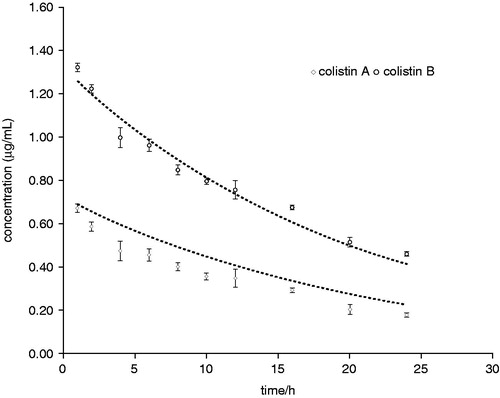
Colistin sulfate and colistimethate sodium are poorly absorbed from the adult gastrointestinal tract, mucosal surfaces, inflamed surfaces or burnsCitation11. Therefore to achieve therapeutic serum level, intramuscular administration of colistimethate sodium must be employed. However, intravenous (i.v.) administration is reported to have a relatively poor Cerebrospinal fluid (CSF) distribution and clinical outcomes varyCitation28. Under different conditions of temperature and time, different proportions of colistimethate sodium are hydrolyzed to colistin. After administration of CMS, colistin appears in plasma rapidly, approximately 50% bound to human plasma. Levels appeared higher but declined more rapidly than those achieved after intramuscular (i.m.) administrationCitation29.
A study performed to assess steady state serum concentrations of colistin after i.v. administration of colistin methanesulfonate (CMS) in critically ill patients revealed CMS dosage regimens administered to them were associated with suboptimal Cmax/minimum inhibitory concentration (MIC) ratios for many strains of Gram-negative bacilli currently reported as sensitive (MIC ≤ 2 mg/L)Citation30. Evidence from PK/PD studies indicates that a colistin loading dose may be beneficial for patients with severe MDR Gram-negative infectionsCitation31. However, the optimal dosing regimen of CMS was not established according to today’s rigorous drug development procedure, for CMS a two-compartment model and for colistin a one-compartment model best described the pharmacokinetics. The half-lives of the two phases for CMS were estimated to be 0.026 and 2.2 h, respectively and for colistin the estimated half-life was 18.5 hCitation32; however, some data estimated it with slight differences (i.e. 14.4 h)Citation33. A reported finding was a colistin plasma peak concentration of 2.93 mg/mL, occurring on average 15 min after the end of the 30 min infusion. However, such an early peak was not observed in the most recent studies, and it can most likely be explained, at least in part, by uncontrolled post-sampling CMS degradation, as only a low percentage of CMS hydrolysis would have a pronounced influence on early colistin concentrationsCitation34. The mean terminal half-life of colistin was 55.7 min. Approximately 60% of the dose was eliminated via the urine in 24 h and presented as a mixture of CMS and colistinCitation21. This was also demonstrated by the rapid initial killing (<2 h) in the time–kill studiesCitation4. In contrast in a previous report on the in vitro pharmacodynamics of colistin against multi-drug-resistant P. aeruginosa, there was significant regrowth at 24 hCitation35. Recent data from PK/PD population studies have suggested that this population could benefit from administration of higher than standard doses of CMS, but the relationship between administration of incremental doses of CMS and corresponding PK/PD parameters as well as its efficacy and toxicity have not yet been investigated in a clinical settingCitation36.
During the first 24 h after dosing, colistin is exerted in the urine, and excessively high serum concentration may therefore occur in patients with renal insufficiencyCitation37. These patients might be expected to metabolize the drug more slowly than those with normal renal function. Studies show that in patients with severe renal failure who are not being dialyzed significant levels of colistin may still be present in the blood two to three days after a single dose of 2–3 mg/kg body weight intravenouslyCitation38. Although PK/PD data in intensive care unit (ICU) patients are scarce, recent evidence shows that the PK and PD of CMS and colistin in critically ill patients differ from those previously found in other groups, such as cystic fibrosis (CF) patientsCitation39.
Spectrum of activity
The antimicrobial spectrum of colistin is narrow; it has in vitro activity against some MDR Gram-negative pathogens, including common or important non-fermentative Gram-negative bacteria, A.baumannii and P. aeruginosaCitation11. The polymyxins have bactericidal activity against most members of the Enterobacteriaceae family includes E. coli, Enterobacter, Salmonella, Shigella and KlebsiellaCitation40. According to the European Committee on Antimicrobial Susceptibility Testing, P. aeruginosa and A. baumannii susceptibilities are defined as MICs of ≤4 and ≤2 mg/L colistin sulfate, respectivelyCitation41, and according to the Clinical and Laboratory Standards Institute (CLSI) as an MIC of ≤2 mg/L for both bacteriaCitation37.
On the other hand eukaryotic microbes and mammalian cells, Proteus species, Neisseria species, Serratia species, Providencia species, Burkholderia pseudomallei, Morganella morganii, and Edwardsiella tarda as well as anaerobic bacteria are typically resistant to colistinCitation42–47. The polymyxins also demonstrated no activity against Gram-negative and Gram-positive cocci and Gram-positive bacilliCitation40. Stenotrophomonas maltophilia and Aeromonas species (except A. jandaei) are susceptible, although A. hydrophila has inducible resistanceCitation48,Citation49.
Mechanism of action
The bactericidal effect of colistin is extremely rapid but the exact mechanism by which colistin can kill bacterial cells is currently unclear. Between colistin and polymyxin B, colistin is used more extensively worldwide due to its better availabilityCitation50. Colistin resistance is not dependent upon bacterial metabolic activity and acquired resistance is rareCitation48. Gram-negative bacteria are characterized by the presence of an outer membrane. The protective function of the outer membrane mainly relies on the presence of lipopolysaccharide (LPS) constituents at the surface of the cell, which limit the penetration of hydrophobic and/or large antibioticsCitation51. The polyanionic bacterial LPS is the initial target, it bears negative charge and this LPS confers to the integrity and stability of the bacterial outer membrane. But polymyxins having positive charge is critical for their interaction with the hydrophobic lipid A component of LPSCitation52,Citation53. Their antibacterial effect on Gram-negative bacteria acts through a detergent-like effect, via a two-step mechanism. It comprises initial binding with electrostatic interactions between the polycationic ring of colistin to cell envelope components, causing the displacement, in a competitive fashion, of the calcium (Ca2+) and magnesium (Mg2+) ions from the phosphate groups of LPS that act as membrane stabilizers, leading to disruption of the outer membrane and to the loss of cellular contents, thus killing the bacterium ()Citation54–56. This process is independent of the entry of polymyxins into the cellCitation57, and seems to be inhibited in the presence of these bivalent cationsCitation53. The killing process with colistin is not dependent upon bacterial metabolic activity, and this may be a significant contributing factor towards the slow development of resistance, a resistance which develops more slowly than that to tobramycinCitation11.
Figure 3. Action of colistin on bacterial membrane. The cationic cyclic decapeptide structure of colistin binds with the anionic LPS molecules by displacing calcium and magnesium from the outer cell membrane of Gram-negative bacteria, leading to permeability changes in the cell envelope and leakage of cell contents. By binding to the lipid A portion of LPS, colistin also has an anti-endotoxin activity. Disruption of the membranes should promote permeability for more conventional anti-pseudomonals. LPS: lipopolysaccharides; PBP: penicillin-binding protein. (From Martis et al.Citation58).
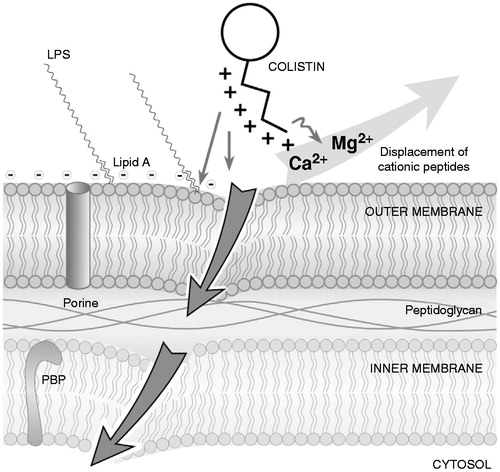
Besides leading to cytoplasmic leakage, this binding can have a neutralizing effect on the biological properties of endotoxinsCitation25. The endotoxin of Gram-negative bacteria is the lipid A portion of LPS molecules, and colistin binds and neutralizes LPS. The significance of this mechanism for in vivo antimicrobial action, namely prevention of the endotoxin’s ability to induce shock through the release of cytokines, is not clear, because plasma endotoxin is immediately bound by LPS-binding protein, and the complex is quickly bound to cell-surface CD14Citation42. More recently, the effects of slow-releasing colistin microspheres suggest that higher blood concentrations of colistin reduce the levels of endotoxin and cytokines in endotoxin-induced sepsis, and lead to decreased toxicityCitation58. An alternative mechanism has recently been presented, demonstrating that polymyxins induce rapid cell death through hydroxyl radical production. Notably, it was demonstrated that this mechanism of killing occurs in multi-drug-resistant clinical isolates of A. baumannii and that this response is not induced in a polymyxin-resistant isolateCitation59.
Mechanisms underlying colistin resistance
Unfortunately, colistin does not escape development of resistance. Although the incidence of colistin resistance is currently relatively rare worldwide, likely reflecting the uncommon use of colistin, colistin-resistant isolates have recently been identified in several GNB species such as A. baumannii, K. pneumoniae and P. aeruginosaCitation60. It is important that the colistin-resistant clones do not spread to non-infected people since colistin is an important antibiotic for eradication of initial and intermittent P. aeruginosa colonizationCitation61.
Although the mechanism underlying resistance is unclear, resistance to the colistin as other polymyxins family has several molecular mechanisms that has been characterized in various bacterial species. It has been suggested resistance to this antibiotic is related to LPS modification via diverse routes. These include: (i) specific modification of outer membrane porins and reductions in the overall negative charge of the LPS, (ii) overexpression of efflux pump systems, (iii) overproduction of capsule polysaccharideCitation62, and (iv) although enzymatic mechanisms of resistance haven’t been reported so far, strains of B. polymyxa are known to produce colistinaseCitation63.
Colistin resistance in Gram-negative bacteria is most commonly due to decreased binding to the bacterial outer membrane because of lipopolysaccharide remodeling that is caused by changes in PhoPQ and PmrAB, both two-component regulatory systems, which results in a less anionic lipid A, which stop or reduce this initial interaction with a different mechanism ()Citation64,Citation65. The pmr locus is an auto-regulated two-component signal transduction system, which in addition to a sensor kinase and response regulator, also includes an ethanolamine transferase. The ethanolamine transferase contributes to colistin resistance by adding ethanolamine moieties to the lipid A component of LPS, which reduces the negative charge of the bacterial membrane and thereby decreases binding of positively charged colistinCitation66. The expression pattern of two-component regulatory systems in the presence of colistin was different from that in the absence of colistin: overall, pmrAB, pmrD, and phoPQ expressed the highest at the stationary phase in the absence of colistin, but the expression of pmrD and phoPQ decreased with the growth in the amount of colistinCitation67. In many Gram-negative bacteria, acquired polymyxin resistance is most often mediated by replacement of lipid A by addition of 4-amino-4-deoxy-L-arabinose (L-Ara4N) and/or phosphoethanolamine (PEtn)Citation55. This action requires the products of the ugd and pbg loci and ethanolamine, mediated by pmrC, therefore these modifications remove negative charges, lowering the affinity of LPS, thereby increasing resistance to polymyxinsCitation68. In addition, a non-specific mechanism for the tolerance of the metabolically active cells to colistin was shown to be up-regulation of the MexAB-OprM efflux pumpCitation69. According to the CLSI an MIC of ≤2 mg/L is susceptible and an MIC of ≥4 mg/L is resistant for Acinetobacter spp., which is different from P. aeruginosa (≥8 mg/L) and other non-EnterobacteriaceaeCitation70,Citation71.
Figure 4. PmrA/PmrB and PhoP/PhoQ two-component regulatory systems. (From Falagas et al.Citation53).
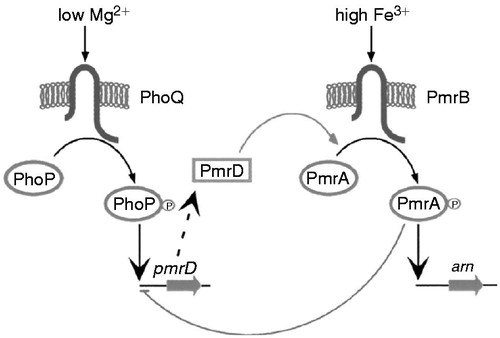
Colistin resistance in A. baumannii
According to data colistin resistance is relatively rare in A. baumannii, and little is known about its mechanism. Moffatt et al. in their study reported spontaneously occurring, lipid A-deficient, Gram-negative bacterial mutants. Moreover, they provided evidence that loss of LPS can lead to polymyxin resistance, that loss of LPS production in A. baumannii can result from inactivation of a lipid A biosynthesis gene, lpxA, lpxC, or lpxD, and that colistin resistance via loss of lipid A can occur in clinical isolatesCitation55. These authors recently showed that insertion sequence ISAba11 movement can result in inactivation of the A. baumannii lipid A biosynthesis genes lpxA and lpxC, resulting in the complete loss of lipopolysaccharide production and high-level colistin resistanceCitation72. Loss or decreased expression of OmpW porin was also reported as a possible colistin resistance mechanism in the A. baumannii mutantCitation73. Resistance can arise through mutations in the two component system pmrA and pmrB, in which the downstream target PmrC catalyzes the addition of PEtn to the lipid A component of LPSCitation74. Park et al. also indicated that increased expression of the PmrAB system is essential for colistin resistance in A. baumannii but that amino acid alterations might be only partially responsible for resistanceCitation60. Although PmrAB and PhoPQ play an important role in colistin resistance in other bacteria such as S. enterica and P.aeruginosa, PhoPQ is absent in A. baumanniiCitation60,Citation75.
Colistin resistance in P. aeruginosa
Although a major route of adaptive polymyxin resistance involves the multi-component regulatory genes PmrAB, PhoPQ, ParRS and CprRS, which are known to be involved in polymyxin resistance in P. aeruginosa, the precise molecular details of these resistance mechanisms have also remained unclear, including a correlation of polymyxin susceptibility with amino acid alterations in these two-component systemsCitation76,Citation77. However, expression of the ParRS and CprRS genes was not consistentCitation78. The PmrAB system responds to limiting Mg2+, and is affected by a phoQ, but not a phoP mutation. Inactivation of the pmrB sensor kinase and pmrA response regulator greatly decreased the expression of the operon encoding pmrA–pmrB while expression of the response regulator pmrA in trans resulted in increased activation suggesting that the pmrA–pmrB operon is autoregulatedCitation79. The PhoPQ system is a global regulatory system that autoregulates the oprH–phoP–phoQ operon under divalent cation-limiting conditions and in the presence of polyamines, which induce resistance to cationic peptides such as colistinCitation80. Data from Lee et al. indicated that amino acid substitutions of PmrAB or PhoPQ do not have an immediate connection with decreased susceptibility of colistin in P. aeruginosa isolates, although activated expression of pmrAB and/or phoPQ resulting in overexpression of pmrH may be required for colistin resistanceCitation78. PmrAB directly controls the pmr HIJKLM operon, whose gene products are responsible for the synthesis of N4-aminoarabinose, which binds to lipid A moieties and neutralizes the negatively charged phospholipids, causing a change in the negatively charged cell membrane which leads to colistin resistanceCitation80.
Colistin resistance in Enterobacteriaceae
The expression of the L-Ara4N and PEtn transferases in E. coli and Salmonella enterica is also regulated by the two component regulatory system PmrA/PmrB, which sensing environmental properties such as pH, Fe3+ and Mg2+ level, as well as the presence of polymyxins, leading to altered expression of a set of genes involved in lipid A modificationCitation9,Citation55.
Salmonella typhimurium regulates mechanisms of resistance to cationic antimicrobial peptides through the two-component systems PhoP–PhoQ and PmrA–PmrBCitation81. Polymyxin resistance is encoded by the PmrA–PmrB regulon, whose products modify the LPS core and lipid A regions with ethanolamine and add aminoarabinose to the 4 phosphate of lipid ACitation81. Mutations in the pmrA locus of S. typhimurium confer an increase in resistance to polymixins. In addition, pmrA mutants survive better in human neutrophils, suggesting that this locus plays a role in virulenceCitation82. Polymyxin-resistant mutants of S. typhimurium and E. coli have a higher substitution of the ester-linked phosphate group in the lipid A portion of the LPS by 4-amino-4-deoxy-L-arabinose and show larger amounts of 2-aminoethanol esterifying phosphates in the core oligosaccharideCitation83. The 4-aminoarabinose substitution is almost stoichiometric in strains of Proteus mirabilis, Chromobacterium violaceum, and Burkholderia cepacia that exhibit innate resistance to polymyxinsCitation83.
In KPC-producing K. pneumoniae (KPC-KP) it has been suggested that the mgrB alteration can be a common mechanism of colistin resistance in the clinical settingCitation84. Comparative genomic analysis of a pair of sequential KPC-KP isolates from the same patient including a colistin-susceptible isolate (KKBO-1) and a colistin-resistant isolate (KKBO-4) selected after colistin exposure revealed that insertional inactivation of the mgrB gene, encoding a negative regulator of the PhoQ/PhoP signaling system, is a genetic mechanism for acquired colistin resistanceCitation85. A recent study confirmed the MgrB regulatory role in K. pneumoniae and was in agreement with the known association between upregulation of the PhoQ/PhoP system and activation of the pmr HFIJKLM operon, which eventually leads to resistance to polymyxins by modification of the lipopolysaccharide target.
Antibacterial combination therapy with colistin
The proportion of cells exhibiting heteroresistance from patients treated with colistin is significantly highCitation86. Colistin heteroresistance, which has been identified in K. pneumoniae, A. baumannii, and P. aeruginosa, is thought to contribute to the rapid emergence of colistin resistance observed with monotherapyCitation87. In clinical practice, it is frequently used as combination therapy in order to improve its antibacterial activity, despite the consequent increase in toxicityCitation88. As simply increasing polymyxin dosage regimens is not an option for optimizing their PK/PD due to nephrotoxicity, combination therapy with other antibiotics has great potential to maximize the efficacy of polymyxins while minimizing emergence of resistanceCitation18. There have been contradictory reports about the effectiveness of combination therapy versus monotherapy and some studies have rejected it. However, the clinical cure rate and microbiological eradication rate were higher in the colistin combined therapy group than in the colistin monotherapy group (48.6% vs. 42.5% and 66.7% vs. 59.4%, respectively), although the differences were not statistically significantCitation89.
Recently combination of colistin and a bacteriocin (nisin) showed a synergistic effect against Gram-negative bacteria and offers the possibility of eliminating the toxicity of this drug, as evidenced by the experiments carried out with mammalian cellsCitation90. In a study about in vitro activities of colistin combinations against P. aeruginosa isolated from the intensive care unit, synergistic activity of combinations of colistin-azithromycin, colistin-doxicycline and colistin-rifampicin was less than expected and a high percentage of indifferent results was observedCitation91. Colistin resistance seems to promote the in vitro activity of unconventional colistin combination. Little impact was demonstrated on vancomycin, trimethoprim, or trimethoprim–sulfamethoxazole MIC valuesCitation51.
In combination, synergy was observed with rifampicin, imipenem, arbekacin, aztreonam, piperacillin, and ciprofloxacin in strains, and according to data this combination is safe and effective. Maximum synergy was observed with colistin plus rifampicin (synergy, 80.0%; additive, 17.5%)Citation94–94. For pneumonia especially, intranasal colistin with rifampicin may be beneficial not only for synergistic antibacterial activity, but also for blocking LPSCitation92. This combination may have a role in the treatment of multi-drug-resistant K. pneumoniae and may possibly slow the selection of heteroresistant subpopulations during colistin therapyCitation95. Other antimicrobial combinations with carbapenems, gentamicin, and tigecycline also showed variously synergistic resultsCitation95. According to data in critically ill patients with carbapenem-resistant A. baumannii infections, clinical outcomes do not differ in patients treated with colistin plus vancomycin from those receiving colistin without vancomycin and this combination significantly increases the risk of renal failureCitation96. The colistin/sulbactam combination therapy reported is promising in severe MDR A. baumannii VAP (ventilator-associated pneumonia). Although the difference was not statistically significant, clinical cure rates and bacteriological clearance rates were better in the combination group than colistin monotherapyCitation97. Colistin-based combination therapy resulted in significantly higher microbiological eradication rates, relatively higher cure and survival rates, and lower in-hospital mortality compared to colistin monotherapyCitation98.
Colistin combination therapy may be required to treat biofilm-associated infections. Colistin and doripenem in combination against biofilm-embedded and planktonic MDR P. aeruginosa, doripenem enhanced killing by colistin of biofilm-embedded cells in both carbapenem-susceptible and -resistant strains, and the combination minimized the emergence of colistin resistanceCitation99.
Adverse effects
It was reported in the old literature that the use of polymyxins was associated with a high incidence of toxicity, such as nephrotoxicity, neurotoxicity, and neuromuscular blockade, sometimes with fatal consequences. Therefore colistin use was limited in the late 1970s, except for treatment of patients with CFCitation1,Citation100–102. However, recent studies showed that the incidence of nephrotoxicity is less common and severe compared to the old studies. In addition, neurotoxic effects of polymyxins are usually mild and resolve after prompt discontinuation of the antibiotics. Furthermore, cases of neuromuscular blockade and apnea have not been reported in the recent literatureCitation103.
Higher colistin doses, similar to those commonly used in the United States, led to a relatively high rate of nephrotoxicityCitation104. Their nephrotoxicity may complicate the therapy or even require its discontinuation. It was reported that colistin monotherapy was not inferior to colistin combination therapy with respect to cure of the infection and nephrotoxicityCitation105. It is notable that nephrotoxicity due to colistin treatment is more common in patients older than 60 years and is related to low initial glomerular filtration rate (GFR)Citation106, so it is wise that patients receiving intravenous colistin should be monitored for nephrotoxicity.
Additionally, the use of polymyxins has been associated with the experience of several neurotoxic events, including dizziness, muscle weakness, facial and peripheral paresthesia, vertigo, confusion, ataxia and neuromuscular blockade, which can lead to respiratory failure or apnea, convulsions and comaCitation107. Death was attributed to colistin therapy in less than 5% of casesCitation58.
Prevalence of colistin resistance
As has been mentioned, nowadays the prevalence of colistin resistance is low, and it has been reported in a few studies. Increasing use of colistin for MDR-GNB infections has led to the emergence of colistin resistance in several countries worldwide. Prevalence may vary between regions and over time; however, some countries (e.g. Japan and South Africa) haven’t access to colistin and some areas of the world (e.g. Europe, Australia) have only the parenteral formulation of colistin (as CMS), whereas in other areas (e.g. USA, Brazil, Malaysia, and Singapore) clinicians can use either colistin or the polymyxin B parenteral formulationCitation108. Despite the majority of the remaining reports presenting resistance rates under 10%, there are also some studies that report high resistance rates. It should be noted that these variations could exist due to differences in methodology. Some laboratories use disk diffusion, and polymyxins diffuse poorly due to high molecular weight. Hence, one could underestimate resistance when using disk diffusionCitation53.
In a study by Gales et al. from the SENTRY antimicrobial surveillance program, in worldwide collection of Gram-negative pathogens reported resistance to the polymyxins remained stable among organisms tested except for Klebsiella spp. isolates collected from the Asia-Pacific and Latin American regions. Indeed, only 0.4% of P. aeruginosa, 0.9% of Acinetobacter spp. and 1.5% of Klebsiella spp. were resistant to colistinCitation43. Another study in the SENTRY antimicrobial surveillance program in 2008 examined the susceptibility to colistin of multi-drug-resistant A. baumannii from the Western Pacific region. It revealed that all the isolates were highly multi-resistant, and colistin MICs were 0.5–2 mg/L except one isolate which had an MIC of 128 mg/LCitation109.
In addition to colistin resistance, heteroresistance has been identified, which has been observed in several Gram-negative pathogensCitation4,Citation86. Heteroresistance is broadly defined as the presence of an antibiotic-resistant subset of microbes within a larger population that is susceptible to the antibiotic. Heteroresistance can complicate assessment of the MIC to a specific antibiotic and may promote resistance to antibiotics in vivo, thereby affecting diagnostic tests and patient treatmentCitation110. However, because of a lack of uniform standards to determine heteroresistance, the rates from different regions varied greatly.
American countries
Most reports from the USA represent resistance rates for both P. aeruginosa and A. baumannii as less than 5.5%Citation111,Citation112. However, studies of resistance in K. pneumoniae report higher ratesCitation53. Development of resistance to colistin in multi-drug-resistant P aeruginosa has not been observed in Denmark despite the use of nebulized colistimethate sodium in combination with oral ciprofloxacinCitation19. The in vitro activity of colistin evaluated against Gram-negative bacilli from patients in Canadian hospitals during 2007–2008 showed colistin was active in vitro (MIC90, ≤2 mg/L) against a variety of clinically important Gram-negative bacilli, including E. coli, Klebsiella spp., Enterobacter spp., A. baumannii, and P. aeruginosa; also all MDR P. aeruginosa clinical isolates evaluated remained susceptible to colistinCitation113. To identify colistin-resistant A. baumannii isolates in an intensive care unit from Argentina, Colistin heteroresistance was observed in 46 (4%) of these isolates and the majority belonged to clones previously identified as I and IIICitation114. Other countries in South America, including Brazil and Chile, report resistance rates of up to 9% for P. aeruginosa and A. baumanniiCitation53.
African countries
African reports are scarce. Studies from Nigeria and South Africa reported that resistance rates were less than 10% and fully susceptible to colistin, respectivelyCitation115,Citation116. Mezghani Maalej et al. from Tunisia in a retrospective study isolated 121 strains of colistin resistant Enterobacteriaceae from 93 patients. The study reported the rate of resistance to colistin ranged from 0.09% for E. coli to 1.2% for K. pneumoniae, and 1.5% for E. cloacaeCitation117. However, from Zimbabwe cumulative data for P. aeruginosa from two different hospitals reported resistance rates of 53%Citation118.
European countries
There are different reports about the resistance rate in Europe. In the UK a survey of CF patients reported that colistin was clearly the most active antimicrobial tested, with 97% of isolates susceptible to <4 mg/L with susceptibility testing by E-test, and 3.1% of P. aeruginosa isolates were resistant to colistinCitation119.
Arroya et al. in 2005 compared the E-test to the broth microdilution method for testing the colistin susceptibility of 115 clinical isolates of A. baumannii. In their study from Spain twenty-two (19.1%) strains were resistant to colistinCitation120. From Romania the resistance rate of E. coli and Klebsiella spp. were reported to be 11% and 17%, respectivelyCitation121.
Capone et al. during 2010–2011 performed a prospective study of patients with carbapenem-resistant K. pneumoniae isolation, hospitalized in nine hospitals in Rome, Italy. They observed a high rate of resistance to colistin that is independently associated with worse outcome; 36.1% of strains were also resistant to colistinCitation122. They suggested that the increased use of this drug during recent years, especially as monotherapy, could be the cause of this. Mammina et al. also described polyclonal spread of colistin-resistant K. pneumoniae in an acute general hospital in Italy. In their study fifty-two isolates had MICs for colistin of 6–128 mg/L, carried blaKPC3 and were attributed to sequence type ST258Citation123.
In 2003, at least nine isolates were reported non-susceptible to colistin in GreeceCitation124. A retrospective cohort study of colonization and infection by colistin-resistant Gram-negative bacteria in critically ill patients from Athens (Greece) during 2003–2006 revealed that 52% of patients were colonized by colistin-resistant Gram-negative bacteria, of whom 20% were colonized by K. pneumoniae isolates and 34% were colonized by intrinsically resistant to colistin (CIR) enterobacteriaceaeCitation125. Another study from Greece during 2005–2008 reported that 10.5% of Klebsiella pneumonia isolates were non-susceptible to colistin and among the imipenem-resistant isolates 20% were also resistant to colistin although no resistance was observed during 1996–1998Citation126. It was reported that colistin resistance had risen at an alarming rate, from 1% in 2005 to 19% in 2008. In Greece’s study published in 2010, all isolates were resistant to carbapenems, β-lactams, ciprofloxacin, aminoglycosides and colistin, but intermediately susceptible to tigecycline and susceptible to gentamicinCitation127.
Resistance to this drug in K. pneumoniae has been extensively described in Hungary. During 2008–2009, in the north-eastern region of Hungary, nine K. pneumoniae isolates showed non-susceptibility to carbapenems, and of these, eight isolates were highly resistant to colistin, which isolates carried blaKPC-2, blaSHV-12, blaTEM-1 and blaSHV-11Citation128. Nowadays epidemiological studies carried out in European countries also observe high colistin use in animal healthCitation129.
Asian countries
Studies from the Asia-Pacific region show that resistance to colistin is common in Enterobacter spp. and has also been detected in Klebsiella spp., and frequently manifests a heteroresistance pattern. In a study resistance to colistin was detected in 0.3% Klebsiella spp. and 21% Enterobacter spp.; however, resistance was seen in all countries except Singapore, ranging from 13.8% (India) to 50% (Philippines)Citation130. In a study from Singapore, 30% of P. aeruginosa isolates were resistant to colistin. In the same study all Acinetobacter spp. and Escherichia coli were susceptible to colistin, and resistance was detected predominantly in S. maltophilia and P. aeruginosa, but was also present in Enterobacter spp. and Klebsiella spp.Citation40. A surveillance study of carbapenem-resistant Enterobacteriaceae isolates from Shanghai (China)Citation131 noticed independent emergence of colistin resistance in KPC-producing carbapenem-resistant Enterobacteriaceae (CRE) isolates without clinical treatment with colistin. In a recent study from China, susceptibility rates for colistin were 92.7%. Although colistin was found to be most active against CRE isolates, four isolates showed high resistance to colistin, with MICs of >64 mg/L for three isolates and 4 mg/L for one isolateCitation131. Another study from China reported that of 112 non-repetitive clinical isolates of A. baumannii–A.calcoaceticus complex, 80% were resistant to a variety of structurally unrelated antimicrobials and resistance to carbapenems occurred in 8% of the isolates, although all isolates were susceptible to polymyxinCitation132.
Iranian data revealed that the rate of colistin resistant A. baumannii with the E-test method was 11.6%. So, as the frequency of resistance to colistin is low, it can be used as an easily available drug for treatment of MDR A. baumannii strains, which are susceptible to colistinCitation133. But in another study on 91 A. baumannii isolates from patients in tertiary intensive care units of three university hospitals in north, central, and south Iran, the drug resistance pattern showed that 14.2%, 20%, and 77% of the A. baumannii isolates were resistant to colistin, tigecycline, and rifampicin, respectivelyCitation134.
According to recent data, high colistin resistance rates in Acinetobacter spp. were reported in Korean hospitals. Ko et al. identified that 18.1% and 27.9% of A. baumannii strains were resistant to polymyxin B and colistin, respectively, belonging to subgroups II and III in Korea, on the basis of rpoB gene analysisCitation135. Multilocus sequence typing (MLST) analysis indicated that most colistin-resistant Acinetobacter spp. isolates from Korean hospitals arose independentlyCitation136. In another study conducted in South Korean hospitals between 2006 and 2007 on patients with bacteremia, 6.8% of K. pneumoniae isolates were resistant to colistinCitation137. In a recent study the clonality of colistin-resistant K. pneumoniae (CRKP) isolates was assessed by MLST. MLST also showed that CRKP isolates were nonclonal, with colistin resistance in K. pneumoniae occurring independently and not by clonal spreading. Studies conducted in Turkey showed all A. baumannii strains were 100% susceptible to colistinCitation102,Citation138–140. However, a study on A. baumannii from Turkey reported resistance rates of 27.5%Citation141. Although colistin appears to be a good choice, adverse reactions and unavailability of colistin limit its wide usage in TurkeyCitation140.
Studies from Thailand reported that colistin is a good option for treatment of MDR P. aeruginosaCitation142,Citation143. In one study colistin had good efficacy against MDR P. aeruginosa with a 98% susceptibility rate and MIC50 and MIC90 for colistin reported to be 1.0 and 1.5 mg/L, respectively, except that two MDR P. aeruginosa isolates were resistant to colistin with MICs of 3 and 12 mg/L, respectivelyCitation143. Moreover, 7.3% colistin resistance for K. pneumonia was reported from ThailandCitation144. In India a high prevalence of resistance was observed in both P. aeruginosa and Acinetobacter isolates to most antibiotics except to colistin, including carbapenems, with rates ranging from 22.1–57.9% and 42–94.0%, respectivelyCitation145. However, 38.3% of P. aeruginosa in 2008 has shown resistant to colistinCitation146. In this country it was also reported that all Metallo-beta-lactamase (MBL)-producing P. aeruginosa producers were susceptible to colistin with MIC ranging from 0.5–0.032 mg/LCitation147. In another study in the case of Acinetobacter spp. which also conducted in North India, 3.5% (from 224 isolates) were resistant to both tigecycline and colistinCitation148. In Israel in a population of injured military personnel returning from Iraq and Afghanistan, the most active agents (>95% of isolates susceptible) were colistin, polymyxin B, and minocyclineCitation149,Citation150.
Conclusion
Colistin is a cationic antimicrobial peptide, with a narrow spectrum of activity mainly against Gram-negative bacteria, that returned to clinical practice due to the lack of options for the treatment of MDR-GNB infections. However, its adverse effects may complicate therapy or even require its discontinuation. It acts by disrupting the bacterial membranes through anionic displacement of stabilizing magnesium and calcium, resulting in leakage of cell contents and eventually in cellular death. Increasing use of colistin for treatment of infections caused by these bacteria has led to the emergence of colistin resistance in several countries worldwide. The highest resistance rate was reported in Asia (especially Korea and Singapore), followed by Europe (especially Greece) and America, where colistin resistance rates are continually increasing. In summary, our review demonstrated that colistin is highly useful as a preferred alternative agent and it was at least as effective as or even more effective than β-lactams, quinolones, and aminoglycosides in the treatment of MDR-GNB patients.
Transparency
Declaration of funding
This study was supported by a grant from the Drug Applied Research Center (Tabriz University of Medical Sciences) with Grant No. 93007782.
Declaration of financial/other relationships
A.Z.B. and H.S.K. have disclosed that they were recipients of the research grant named above.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank all staff of educational hospitals for cooperation in antibiotic stewardships of Tabriz University of Medical Science. They also thank Dr. Hossein Navidinia for his helpful comments on manuscript.
References
- Kasiakou SK, Michalopoulos A, Soteriades ES, et al. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother 2005;49:3136-46
- Spellberg B, Blaser M, Guidos RJ, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 2011;52(Suppl 5):S397-428
- Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 2014;59:88-94
- Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2006;50:2946-50
- Hsueh PR, Tseng SP, Teng LJ, Ho SW. Pan-drug-resistant Pseudomonas aeruginosa causing nosocomial infection at a university hospital in Taiwan. Clin Microbiol Infect 2005;11:670-3
- Littlewood JM, Koch C, Lambert PA, et al. A ten year review of colomycin. Respir Med 2000;94:632-40
- Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006;50:1953-8
- Velkov T, Thompson PE, Nation RL, Li J. Structure–activity relationships of polymyxin antibiotics. J Med Chem 2009;53:1898-916
- Adams MD, Nickel GC, Bajaksouzian S, et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother 2009;53:3628-34
- Bassetti M, Repetto E, Righi E, et al. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J Antimicrob Chemother 2008;61:417-20
- Li J, Nation RL, Milne RW, et al. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 2005;25:11-25
- Rios FG, Luna CM, Maskin B, et al. Ventilator-associated pneumonia due to colistin susceptible-only microorganisms. Eur Respir J 2007;30:307-13
- Gunderson BW, Ibrahim KH, Hovde LB, et al. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2003;47:905-9
- Falagas ME, Michalopoulos A. Polymyxins: old antibiotics are back. Lancet 2006;367:633-34
- Brink AJ, Richards GA, Colombo G, et al. Multicomponent antibiotic substances produced by fermentation: implications for regulatory authorities, critically ill patients and generics. Int J Antimicrob Agents 2014;43:1-6
- Li J, Milne RW, Nation RL, et al. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother 2002;46:3304-7
- Dhariwal AK, Tullu MS. Colistin: re-emergence of the ‘forgotten' antimicrobial agent. J Postgrad Med 2013;59:208-15
- Bergen PJ, Landersdorfer CB, Zhang J, et al. Pharmacokinetics and pharmacodynamics of ‘old' polymyxins: what is new? Diagn Microbiol Infect Dis 2012;74:213-23
- Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006;6:589-601
- Li J, Milne RW, Nation RL, et al. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother 2003;47:1364-70
- Li J, Milne RW, Nation RL, et al. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 2004;53:837-40
- Beveridge EG, Martin AJ. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother 1967;29:125-35
- Schwartz BS, Warren MR, Barkley FA, Landis L. Microbiological and pharmacological studies of colistin sulfate and sodium colistinmethanesulfonate. Antibiotics Annual 1959;7:41-60
- Boisson M, Gregoire N, Couet W, Mimoz O. Colistin in critically ill patients. Minerva Anestesiologica 2013;79:200-8
- Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev 2008;21:449-65
- Vaara M, Vaara T. Structure–activity studies on novel polymyxin derivatives that carry only three positive charges. Peptides 2010;31:2318-21
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011;55:3284-94
- Ziaka M, Markantonis SL, Fousteri M, et al. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother 2013;57:1938-40
- Froman J, Gross L, Curatola S. Serum and urine levels following parenteral administration of sodium colistimethate to normal individuals. J Urol 1970;103:210-14
- Markou N, Markantonis SL, Dimitrakis E, et al. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Therapeut 2008;30:143-51
- Wertheim H, Van Nguyen K, Hara GL, et al. Global survey of polymyxin use: a call for international guidelines. J Global Antimicrob Resist 2013;1:131-4
- Mohamed AF, Karaiskos I, Plachouras D, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 2012;56:4241-9
- Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009;53:3430-6
- Couet W, Gregoire N, Marchand S, Mimoz O. Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect 2012;18:30-9
- Li J, Turnidge J, Milne R, et al. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother 2001;45:781-5
- Luque S, Grau S, Valle M, et al. Differences in pharmacokinetics and pharmacodynamics of colistimethate sodium (CMS) and colistin between three different CMS dosage regimens in a critically ill patient infected by a multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents 2013;42:178-81
- MacKay DN, Kaye D. Serum concentrations of colistin in patients with normal and impaired renal function. N Engl J Med 1964;270:394-97
- Curitis JR, Eastwood JB. Colistin sulphomethate sodium administration in the presence of severe renal failure and during haemodialysis and peritoneal dialysis. Br Med J 1968;1:484-5
- Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care 2011;1:1-6
- Tan T, Ng SY. The in-vitro activity of colistin in gram-negative bacteria. Singapore Med J 2006;47:621-4
- Renard L, Sanders P, Laurentie M. [Pharmacokinetics of colistin sulfate administered by intravenous and intramuscular routes in the calf]. Ann Vet Res 1990;22:387-94
- Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005;40:1333-41
- Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J Antimicrob Chemother 2011;66:2070-4
- Vaara M. Polymyxins and their novel derivatives. Curr Opin Microbiol 2010;13:574-81
- Muyembe T, Vandepitte J, Desmyter J. Natural colistin resistance in Edwardsiella tarda. Antimicrob Agents Chemother 1973;4:521-4
- Shimizu S, Iyobe S, Mitsuhashi S. Inducible high resistance to colistin in Proteus strains. Antimicrob Agents Chemother 1977;12:1-3
- Boisson M, Gregoire N, Couet W, Mimoz O. Colistin in critically ill patients. Minerva Anestesiologica 2013;79:200-8
- Fosse T, Giraud-Morin C, Madinier I. Induced colistin resistance as an identifying marker for Aeromonas phenospecies groups. Lett Applied Microbiol 2003;36:25-9
- Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 2012;18:18-29
- Lee CS, Doi Y. Therapy of infections due to carbapenem-resistant gram-negative pathogens. Infect Chemother 2014;46:149-64
- Vidaillac C, Benichou L, Duval RE. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 2012;56:4856-61
- Beceiro A, Moreno A, Fernandez N, et al. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother 2014;58:518-26
- Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updates 2010;13:132-8
- Mendes CA, Burdmann EA. [Polymyxins – review with emphasis on nephrotoxicity]. Revista da Associacao Medica Brasileira 2009;55:752-9
- Moffatt JH, Harper M, Harrison P, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 2010;54:4971-7
- Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother 1999;33:960-7
- Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin Microbiol Infect 2006;12:315-21
- Martis N, Leroy S, Blanc V. Colistin in multi-drug resistant Pseudomonas aeruginosa blood-stream infections: a narrative review for the clinician. J Infect 2014;69:1-12
- Sampson TR, Liu X, Schroeder MR, et al. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother 2012;56:5642-9
- Park YK, Choi JY, Shin D, Ko KS. Correlation between overexpression and amino acid substitution of the PmrAB locus and colistin resistance in Acinetobacter baumannii. Int J Antimicrob Agents 2011;37:525-30
- Johansen HK, Moskowitz SM, Ciofu O, et al. Spread of colistin resistant non-mucoid Pseudomonas aeruginosa among chronically infected Danish cystic fibrosis patients. J Cystic Fibrosis 2008;7:391-7
- Kim Y, Bae IK, Lee H, et al. In vivo emergence of colistin resistance in Acinetobacter baumannii clinical isolates of sequence type 357 during colistin treatment. Diagn Microbiol Infect Dis 2014;79:362-6
- Ito-Kagawa M, Koyama Y. Selective cleavage of a peptide antibiotic, colistin by colistinase. J Antibiotics 1980;33:1551-5
- Lopez-Rojas R, Dominguez-Herrera J, McConnell MJ, et al. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J Infect Dis 2011;203:545-8
- Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 2007;60:1206-15
- Snitkin ES, Zelazny AM, Gupta J, et al. Genomic insights into the fate of colistin resistance and Acinetobacter baumannii during patient treatment. Genome Res 2013;23:1155-62
- Kim SY, Choi HJ, Ko KS. Differential expression of two-component systems, pmrAB and phoPQ, with different growth phases of Klebsiella pneumoniae in the presence or absence of colistin. Curr Microbiol 2014;69:37-41
- Beceiro A, Llobet E, Aranda J, et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 2011;55:3370-9
- Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol 2008;68:223-40
- Peter H, Berggrav K, Thomas P, et al. Direct detection and genotyping of Klebsiella pneumoniae carbapenemases from urine by use of a new DNA microarray test. J Clin Microbiol 2012;50:3990-8
- Moskowitz SM, Garber E, Chen Y, et al. Colistin susceptibility testing: evaluation of reliability for cystic fibrosis isolates of Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother 2010;65:1416-23
- Moffatt JH, Harper M, Adler B, et al. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother 2011;55:3022-4
- Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J 2011;52:879-91
- Lean SS, Suhaili Z, Ismail S, et al. Prevalence and genetic characterization of carbapenem- and polymyxin-resistant acinetobacter baumannii isolated from a tertiary hospital in Terengganu, Malaysia. ISRN microbiology 2014:1-9
- Adams MD, Goglin K, Molyneaux N, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol 2008;190:8053-64
- Haagensen JA, Klausen M, Ernst RK, et al. Differentiation and distribution of colistin- and sodium dodecyl sulfate-tolerant cells in Pseudomonas aeruginosa biofilms. J Bacteriol 2007;189:28-37
- Lee JY, Na IY, Park YK, Ko KS. Genomic variations between colistin-susceptible and -resistant Pseudomonas aeruginosa clinical isolates and their effects on colistin resistance. J Antimicrob Chemother 2014;69:1248-56
- Lee JY, Ko KS. Mutations and expression of PmrAB and PhoPQ related with colistin resistance in Pseudomonas aeruginosa clinical isolates. Diagn Microbiol Infect Dis 2014;78:271-6
- McPhee JB, Lewenza S, Hancock RE. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol Microbiol 2003;50:205-17
- Ly NS, Yang J, Bulitta JB, Tsuji BT. Impact of two-component regulatory systems PhoP-PhoQ and PmrA-PmrB on colistin pharmacodynamics in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2012;56:3453-6
- Gunn JS, Lim KB, Krueger J, et al. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol 1998;27:1171-82
- Roland KL, Martin LE, Esther CR, Spitznagel JK. Spontaneous pmrA mutants of Salmonella typhimurium LT2 define a new two-component regulatory system with a possible role in virulence. J Bacteriol 1993;175:4154-64
- Groisman EA, Kayser J, Soncini FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol 1997;179:7040-5
- Cannatelli A, Giani T, D'Andrea MM, et al. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother 2014;58:5696-703
- Cannatelli A, D'Andrea MM, Giani T, et al. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother 2013;57:5521-6
- Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother 2008;52:351-2
- Deris ZZ, Heidi HY, Davis K, et al. The combination of colistin and doripenem is synergistic against Klebsiella pneumoniae at multiple inocula and suppresses colistin resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 2012;56:5103-12
- Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clin Microbiol Infect 2008;14:816-27
- Gu WJ, Wang F, Tang L, et al. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: a systematic review and meta-analysis. Int J Antimicrob Agents 2014;44:477-85
- Naghmouchi K, Baah J, Hober D, et al. Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob Agents Chemother 2013;57:2719-25
- Lopez-Fabal F, Culebras E, Bonilla I, et al. [In vitro activities of colistin combinations against Pseudomonas aeruginosa isolated from the intensive care unit]. Revista Espanola de Quimioterapia 2008;21:189-93
- Aoki N, Tateda K, Kikuchi Y, et al. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother 2009;63:534-42
- Tascini C, Gemignani G, Palumbo F, et al. Clinical and microbiological efficacy of colistin therapy alone or in combination as treatment for multidrug resistant Pseudomonas aeruginosa diabetic foot infections with or without osteomyelitis. J Chemother (Florence, Italy) 2006;18:648-51
- Nagaoka R, Ikawa K, Onodera M, et al. In vitro combined effects of double antibacterial drugs against multidrug-resistant Pseudomonas aeruginosa isolates: comparison among combinations of colistin, arbekacin, aztreonam, rifampicin and piperacillin. Jpn J Antibiot 2014;67:167-74
- Tascini C, Tagliaferri E, Giani T, et al. Synergistic activity of colistin plus rifampin against colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2013;57:3990-3
- Garnacho-Montero J, Amaya-Villar R, Gutierrez-Pizarraya A, et al. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemother 2013;59:225-31
- Kalin G, Alp E, Akin A, et al. Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection 2014;42:37-42
- Batirel A, Balkan II, Karabay O, et al. Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis 2014;33:1311-22
- Lora-Tamayo J, Murillo O, Bergen PJ, et al. Activity of colistin combined with doripenem at clinically relevant concentrations against multidrug-resistant Pseudomonas aeruginosa in an in vitro dynamic biofilm model. J Antimicrob Chemother 2014;69:2434-42
- Hachem RY, Chemaly RF, Ahmar CA, et al. Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob Agents Chemother 2007;51:1905-11
- Koomanachai P, Landersdorfer CB, Chen G, et al. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 2014;58:440-6
- Sinirtas M, Akalin H, Gedikoglu S. Investigation of colistin sensitivity via three different methods in Acinetobacter baumannii isolates with multiple antibiotic resistance. Int J Infect Dis 2009;13:e217-20
- Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 2006;10:R27
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011;53:879-84
- Falagas ME, Rafailidis P, Kasiakou SK, et al. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin–meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect 2006;12:1227-30
- Balkan II, Dogan M, Durdu B, et al. Colistin nephrotoxicity increases with age. Scand J Infect Dis 2014;46:678-85
- Falagas ME, Kasiakou SK, Tsiodras S, Michalopoulos A. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin Med Res 2006;4:138-46
- Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 2014;59:88-94
- Yau W, Owen RJ, Poudyal A, et al. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect 2009;58:138-44
- Napier BA, Band V, Burd EM, Weiss DS. Colistin heteroresistance in Enterobacter cloacae is associated with cross-resistance to the host antimicrobial lysozyme. Antimicrob Agents Chemother 2014;58:5594-7
- Keen EF III, Robinson BJ, Hospenthal DR, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 2010;36:819-25
- Landman D, Bratu S, Alam M, Quale J. Citywide emergence of Pseudomonas aeruginosa strains with reduced susceptibility to polymyxin B. J Antimicrob Chemother 2005;55:954-57
- Walkty A, DeCorby M, Nichol K, et al. In vitro activity of colistin (polymyxin E) against 3,480 isolates of gram-negative bacilli obtained from patients in Canadian hospitals in the CANWARD study, 2007–2008. Antimicrob Agents Chemother 2009;53:4924-6
- Rodriguez CH, Bombicino K, Granados G, et al. Selection of colistin-resistant Acinetobacter baumannii isolates in postneurosurgical meningitis in an intensive care unit with high presence of heteroresistance to colistin. Diagn Microbiol Infect Dis 2009;65:188-91
- Ahmed NH, Baba K, Clay C, et al. In vitro activity of tigecycline against clinical isolates of carbapenem resistant Acinetobacter baumannii complex in Pretoria, South Africa. BMC Res Notes 2012;5:215
- Iroha EO, Kesah CN, Egri-Okwaji MT, Odugbemi TO. Bacterial eye infection in neonates, a prospective study in a neonatal unit. West African J Med 1998;17:168-72
- Mezghani Maalej S, Rekik Meziou M, Mahjoubi F, Hammami A. Epidemiological study of Enterobacteriaceae resistance to colistin in Sfax (Tunisia). Medecine et Maladies Infectieuses 2012;42:256-63
- Igumbor E, Gwanzura L, Chirara M, et al. Antibiotic sensitivity and plasmid profiles of Pseudomonas aeruginosa. Central African J Med 2000;46:296-300
- Pitt TL, Sparrow M, Warner M, Stefanidou M. Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax 2003;58:794-6
- Arroyo LA, Garcia-Curiel A, Pachon-Ibanez ME, et al. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol 2005;43:903-5
- Miftode E, Dorneanu O, Leca D, et al. [Antimicrobial resistance profile of E. coli and Klebsiella spp. from urine in the Infectious Diseases Hospital Iasi]. Revista Medico-Chirurgicala a Societatii de Medici si Naturalisti din Iasi 2008;112:478-82
- Capone A, Giannella M, Fortini D, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 2013;19:E23-30
- Mammina C, Bonura C, Di Bernardo F, et al. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro Surveillance: European Communicable Disease Bulletin 2012;17:1-4
- Giamarellos-Bourboulis E, Sambatakou H, Galani I, Giamarellou H. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J Chemother 2003;15:235-8
- Kontopidou F, Plachouras D, Papadomichelakis E, et al. Colonization and infection by colistin-resistant Gram-negative bacteria in a cohort of critically ill patients. Clin Microbiol Infect 2011;17:E9-11
- Neonakis IK, Samonis G, Messaritakis H, et al. Resistance status and evolution trends of Klebsiella pneumoniae isolates in a university hospital in Greece: ineffectiveness of carbapenems and increasing resistance to colistin. Chemotherapy 2010;56:448-52
- Kontopoulou K, Protonotariou E, Vasilakos K, et al. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J Hosp Infect 2010;76:70-3
- Toth A, Damjanova I, Puskas E, et al. Emergence of a colistin-resistant KPC-2-producing Klebsiella pneumoniae ST258 clone in Hungary. Eur J Clin Microbiol Infect Dis 2010;29:765-9
- Kempf I, Fleury MA, Drider D, et al. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int J Antimicrob Agents 2013;42:379-83
- Turnidge J, Bell J, Jones R. Emergence of colistin-resistant Klebsiella spp., and Enterobacter spp. in the Asia-Pacific (APAC) region: a SENTRY antimicrobial surveillance program report (2006). Conf Antimicrob Agents Chemother American Society for Microbiology, Washington, DC; 2007;2007
- Chen S, Hu F, Zhang X, et al. Independent emergence of colistin-resistant Enterobacteriaceae clinical isolates without colistin treatment. J Clin Microbiol 2011;49:4022-3
- Lin L, Ling BD, Li XZ. Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial-resistant clinical isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus complex. Int J Antimicrob Agents 2009;33:27-32
- Vakili B, Fazeli H, Shoaei P, et al. Detection of colistin sensitivity in clinical isolates of Acinetobacter baumannii in Iran. J Res Med Sci 2014;19(Suppl 1):S67-70
- Bahador A, Taheri M, Pourakbari B, et al. Emergence of rifampicin, tigecycline, and colistin-resistant Acinetobacter baumannii in Iran; spreading of MDR strains of novel international clone variants. Microb Drug Resist (Larchmont, NY) 2013;19:397-406
- Ko KS, Suh JY, Kwon KT, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother 2007;60:1163-7
- Park YK, Jung SI, Park KH, et al. Independent emergence of colistin-resistant Acinetobacter spp. isolates from Korea. Diagn Microbiol Infect Dis 2009;64:43-51
- Suh JY, Son JS, Chung DR, et al. Nonclonal emergence of colistin-resistant Klebsiella pneumoniae isolates from blood samples in South Korea. Antimicrob Agents Chemother 2010;54:560-2
- Ayan M, Durmaz R, Aktas E, Durmaz B. Bacteriological, clinical and epidemiological characteristics of hospital-acquired Acinetobacter baumannii infection in a teaching hospital. J Hosp Infect 2003;54:39-45
- Timurkaynak F, Can F, Azap OK, et al. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int J Antimicrob Agents 2006;27:224-8
- Dizbay M, Altuncekic A, Sezer BE, et al. Colistin and tigecycline susceptibility among multidrug-resistant Acinetobacter baumannii isolated from ventilator-associated pneumonia. Int J Antimicrob Agents 2008;32:29-32
- Eser OK, Ergin A, Hascelik G. [Antimicrobial resistance and existence of metallo-beta-lactamase in Acinetobacter species isolated from adult patients]. Mikrobiyoloji Bulteni 2009;43:383-90
- Tribuddharat C, Tiensasitorn C, Techachaiwiwat W, et al. In vitro activity of polymyxin B and polymyxin E against multi-drug resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J Infect Dis Antimicrob Agents 2003;20:135-7
- Tunyapanit W, Pruekprasert P, Laoprasopwattana K, Chelae S. In vitro activity of colistin against multidrug-resistant Pseudomonas aeruginosa isolates from patients in Songklanagarind Hospital, Thailand. Southeast Asian J Trop Med Publ Health 2013;44:273-80
- Punpanich W, Tantichattanon W, Wongwatcharapaiboon S, Treeratweeraphong V. In vitro susceptibility pattern of cephalosporin-resistant Gram-negative bacteria. J Med Assoc Thailand 2008;91(Suppl 3):S21-7
- Mohanty S, Maurya V, Gaind R, Deb M. Phenotypic characterization and colistin susceptibilities of carbapenem-resistant of Pseudomonas aeruginosa and Acinetobacter spp. J Infection Developing Countries 2013;7:880-7
- Varaiya A, Kulkarni M, Bhalekar P, Dogra J. Incidence of metallo-beta-lactamase-producing Pseudomonas aeruginosa in diabetes and cancer patients. Ind J Pathol Microbiol 2008;51:200-3
- Manoharan A, Chatterjee S, Mathai D. Detection and characterization of metallo beta lactamases producing Pseudomonas aeruginosa. Ind J Med Microbiol 2010;28:241-4
- Taneja N, Singh G, Singh M, Sharma M. Emergence of tigecycline & colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Ind J Med Res 2011;133:681-4
- Hawley JS, Murray CK, Griffith ME, et al. Susceptibility of acinetobacter strains isolated from deployed U.S. military personnel. Antimicrob Agents Chemother 2007;51:376-8
- Hussein K, Sprecher H, Mashiach T, et al. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Contr Hosp Epidemiol 2009;30:666-71
- Zhao M, Cao Y-R, Guo B-N, et al. LC-MS/MS determination of colistin in Mueller-Hinton broth for in vitro pharmacodynamic studies. J Antibiot 2014;67:825-9
