Abstract
Tanshinone II-A (TSN) is the most abundant diterpene quinone isolated from Danshen (Salvia miltiorrhiza), which has been used in treating cardiovascular diseases for more than 2000 years in China. Interest in its versatile protective effects in cardiovascular, metabolic, neurodegenerative diseases, and cancers has been growing over the last decade. TSN is a multi-target drug, whose molecular targets include transcription factors, scavenger receptors, ion channels, kinases, pro- and anti-apoptotic proteins, growth factors, inflammatory mediators, microRNA, and others. More recently, enhanced or synergistic effects can be observed when TSN is used in combination therapy with cardioprotective and anti-cancer drugs. These combination therapy regimens may open new therapeutic avenues for the treatment of various kinds of human diseases.
1. Introduction
Traditional Chinese medicines (TCM), as a representative of alternative and complementary medicine, are commonly used in Asian countries for the treatment of cancer, cardiovascular, cerebrovascular, metabolic, and neurodegenerative diseases. Among the TCM, Danshen (Salvia miltiorrhiza) has drawn extensive attention as effective therapeutics against a number of diseases including atherosclerosis Citation[1], hyperlipidemia Citation[2], hypertension Citation[3], stable angina pectoris Citation[4], myocardial infarction Citation[4], coronary artery disease Citation[1], diabetes Citation[4] and metabolic syndrome Citation[5], as supported by published papers, approved patents and clinical trials in U.S. (). In China, Danshen formulations include Danshen Tablet, Danshen Capsule, and Dantonic Dripping Pill (DP, also referred to as Cardiotonic® Pill). According to the report from Tianjin-based Tasly Pharmaceutical Co., DP, as the most popular Chinese medicinal product in China for treating coronary heart disease, generates 205 million USD annually Citation[6]. DP is a botanical drug which consists of extracts of Danshen (Radix Salviae miltiorrhizae) and San qi (Radix Panax notoginseng) with borneol. A systematic review of randomized controlled trials (RCT) of DP was published in 2010 Citation[6], although all the RCT were conducted in China with Chinese patients and only published in Chinese medical journals. It is the first TCM approved for Phase II and Phase III clinical trials by the U.S. Food and Drug Administration (). In this editorial, we focus on the scientific literature and approved patents on the therapeutic use and molecular targets of tanshinone II-A (TSN), a major bioactive constituent isolated from Danshen. Combination therapy of TSN and other drugs are also discussed.
Table 1. Overview of clinical trials of Danshen and tanshinone II-A.
2. Literature and patent search
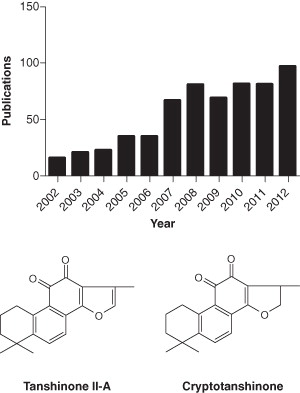
Data were identified through the search of PubMed for research articles and reviews, the websites of European Patent Office and US Patent Office for patents up to October 2012. In total, 607 publications were identified through the electronic database search on PubMed from 2002 to 2012. There is a trend of gradual increase in publications covering tanshinones (). In recent years, many patents have been granted on the isolation, preparation, and pharmacological characterization of tanshinones; diverse TCM formula with Danshen as the major principle; structural modifications of TSN or other lead compounds from Danshen; and emerging new tanshinone derivatives (such as neo-tanshinlactone) for the treatment of cancer Citation[7]. Based on published literature and granted patents, it can be concluded that TSN is an effective therapeutic agent against atherosclerosis Citation[8], hypertension Citation[3], obesity Citation[9], metabolic syndrome Citation[9], and other diseases.
3. Tanshinone II-A
Lipophilic tanshinones isolated from Danshen consist of 0.29% tanshinone II-A (TSN), 0.23% cryptotanshinone (CPT), 0.11% tanshinone I, and 0.054% 15, 16-dihydrotanshinone-I Citation[5]. Of all of these tanshinones that have been identified, TSN and CPT have received the most attention, in that they are the most abundant and well-studied constituents of Danshen which exerts antioxidant and anti-inflammatory actions in many experimental animal models. The chemical structure of TSN and CPT are illustrated in . Over the past decade, we Citation[1,10-19] and others Citation[20-24] have demonstrated that TSN has potential protective effects against atherosclerosis, cardiac hypertrophy, cardiac fibrosis, diabetes, neurodegenerative diseases, and various kinds of cancers. However, the poor water-solubility, poor intestinal absorption, and low oral bioavailability (about 2.9 – 3.4% in rats) Citation[25] have hampered the clinical application of TSN. To overcome these problems, various approaches have been employed, such as the preparation of water-soluble derivative of TSN-sodium tanshinone II-A sulfonate (STS) Citation[4], the preparation of TSN in discoidal and spherical HDL Citation[26], and the development of new drug delivery systems for TSN such as solid dispersion pellets, nanoparticles, and microemulsions Citation[26].
4. Molecular targets of tanshinone II-A
Studies in the past decade suggest that TSN hits the model of “one-drug-multi-target-multi-disease, which is shared by” TCM, such as berberine Citation[27]. Based on published literature and granted patents, we summarized the molecular targets of TSN in . These targets, though some are not characterized in detail, will furnish the molecular basis for the clinical application of TSN to treat various kinds of diseases.
Table 2. Molecular targets of tanshinone II-A
5. Combination therapy
Combination therapy of bioactive herbal ingredients with Western medicine represents a new emerging field in the management of human diseases. Combined use of TSN with other drugs with different mechanisms of action can potentiate the pharmacological effects; reduce side-effects through decreasing the doses of both agents and improve pharmacodynamic and pharmacokinetic properties of drugs. Following combination strategies may be applied in clinical studies in future.
5.1 Tanshinone II-A/statins
We have previously filed a patent application describing the preventive effect of TSN against atherosclerosis in high-fat-diet-fed rabbits Citation[8]. Considering that TSN does not affect lipid metabolism Citation[10-12,14], but has hepatoprotective properties Citation[28,29], Xu et al. described the preparation of a pharmaceutical composition containing TSN and HMG-CoA reductase Citation[30]. However, the pharmacological effects of this combination strategy remain unknown. More recently, we observed that, in athero-prone ApoE knockout mice fed a high-cholesterol-diet, TSN and atorvastatin in combination can reduce and stabilize atherosclerotic plaques in synergism, for the atheroprotective mechanisms of TSN (lipid-independent) and atorvastatin (lipid-lowering) are different Citation[31]. The safety profile and favorable results demonstrated in this combination therapy support its use not only in patients with atherosclerosis who don't tolerate statins, but also in those who don't achieve therapeutic goals with single therapy with statins. In addition, TSN/atorvastatin combination therapy has the potential to overcome the unwanted side-effects of statins (such as life-threatening rhabdomyolysis and liver damage).
5.2 Tanshinone II-A/Angiotensin II-type I receptor blockers
Oral administration of TSN decreased systolic blood pressure of spontaneously hypertensive rats (SHRs) Citation[32]. In contrast, we fail to observe the blood pressure-lowering effect of TSN in stroke-prone 2-kidney-2-clip renohypertensive rats Citation[15,16]. Consistent with our reports, Li et al. Citation[33] observed that TSN prevents left ventricular hypertrophy in the myocardium of hypertensive rats with abdominal aorta constriction, independent of blood pressure. Therefore, it is conceivable that TSN and angiotensin II-type I receptor blockers (ARBs) may have a synergistic protective effects on patients with hypertension.
5.3 Other combination strategies
P-glycoprotein (P-gp) functions as a drug export pump that decreases intracellular concentrations of chemotherapeutic agents. TSN is a potential P-gp inhibitor during chemotherapy as it potentiates the efficacy of 5-FU in a colon cancer nude SCID mouse model Citation[34]. Furthermore, TSN can be used in combination with other bioactive constituents from TCM. Wang et al. Citation[35] recently disclosed a patent describing a medicinal composition of tanshinone II-A sulfate and ferulic acid to treat cardiovascular and cerebrovascular diseases.
6. Expert opinion
Although substantial progress has been made in elucidating the cellular and molecular targets of TSN, it remains unknown which are direct targets as well as the mechanisms of its multi-targeted actions. Preclinical studies have clearly demonstrated the efficacy of TSN in experimental animal models. Such information will be essential in translating the beneficial effects into clinical practice. At present, several clinical trials with TSN are already ongoing or completed in U.S. and we are waiting to see whether TSN (either used alone or in combination with other drugs) can be a therapeutic agent. Combining all available data, we expect that TSN could be considered as an effective therapeutic agent against various kinds of diseases, also it may serve as the lead compound or novel drug candidate in the battle against various kinds of human diseases.
Declaration of interest
The authors state no other conflicts of interest and have received no payment in preparation of this manuscript.
Acknowledgments
The authors gratefully acknowledge the contribution of investigators dedicated to the isolation, preparation, and pharmacological characterization of tanshinones and their derivatives, and we apologize to those investigators whose work we could not cite due to reference limit. This work was supported by research grants from the National Natural Science Foundation of China (No. 81072641, No. 81273499), the National Science and Technology Major Project of China “Key New Drug Creation and Manufacturing Program” (No. 2011ZX09401-307) Team Item of Natural Science Foundation of Guangdong Province (No. S2011030003190), Major Project of Guangdong Province (No. 2008A030201013, No. 2012A080201007), Major Project of Department of Education of Guangdong Province (No.CXZD1006), and ‘‘New Investigator Award” from Ministry of Education of China.
Bibliography
- Gao S, Liu Z, Li H, Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 2012;220(1):3-10
- Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005;45(12):1345-59
- Wang J, Lu W, Chen Y, Application of tanshinone IIA or pharmaceutically acceptable salts thereof in preparing medicines for treating or preventing pulmonary hypertension disease. CN102160866; 2011
- Shang Q, Xu H, Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med 2012;2012:716459
- Taehwan K, Myunggyu P. Obesity and metabolic syndrome treatment with tanshinone derivatives which increase metabolic activity. US8029832; 2011
- Jia Y, Huang F, Zhang S, Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. Int J Cardiol 2012;157(3):330-40
- Lee KH, Wang X, Bastow K, Neo-tanshinlactone and analogs as potent and selective anti-breast cancer agents. US7795299 B2; 2010
- Gu L, Liu P, Li G, Tanshinone IIA for prophylaxising or treating atherosclerosis. WO2004058245; 2004
- Kwak TH, Park MG. Obesity and metabolic syndrome treatment with tanshinone derivatives which increase metabolic activity. KR20080015495; 2008
- Tang F, Wu X, Wang T, Tanshinone II A attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vascul Pharmacol 2007;46(6):427-38
- Tang FT, Cao Y, Wang TQ, Tanshinone IIA attenuates atherosclerosis in ApoE(-/-) mice through down-regulation of scavenger receptor expression. Eur J Pharmacol 2011;650(1):275-84
- Xu S, Little PJ, Lan T, Tanshinone II-A attenuates and stabilizes atherosclerotic plaques in apolipoprotein-E knockout mice fed a high cholesterol diet. Arch Biochem Biophys 2011;515(1-2):72-9
- Xu S, Liu Z, Huang Y, Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-kappaB activation. Transl Res 2012;160(2):114-24
- Chen W, Tang F, Xie B, Amelioration of atherosclerosis by tanshinone IIA in hyperlipidemic rabbits through attenuation of oxidative stress. Eur J Pharmacol 2012;674(2-3):359-64
- Fang J, Xu SW, Wang P, Tanshinone II-A attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine 2010;18(1):58-64
- Wang P, Wu X, Bao Y, Tanshinone IIA prevents cardiac remodeling through attenuating NAD(P)H oxidase-derived reactive oxygen species production in hypertensive rats. Pharmazie 2011;66(7):517-24
- Fu J, Huang H, Liu J, Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol 2007;568(1-3):213-21
- Gao J, Yang G, Pi R, Tanshinone IIA protects neonatal rat cardiomyocytes from adriamycin-induced apoptosis. Transl Res 2008;151(2):79-87
- Xu S, Ogura S, Chen J, LOX-1 in Atherosclerosis: biological Functions and Pharmacological Modifiers. Cell Mol Life Sci 2012; DOI: 10.1007/s00018-012-1194-z
- Dong Y, Morris-Natschke SL, Lee KH. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat Prod Rep 2011;28(3):529-42
- Gong Z, Huang C, Sheng X, The role of tanshinone IIA in the treatment of obesity through peroxisome proliferator-activated receptor gamma antagonism. Endocrinology 2009;150(1):104-13
- Fang ZY, Lin R, Yuan BX, Tanshinone IIA inhibits atherosclerotic plaque formation by down-regulating MMP-2 and MMP-9 expression in rabbits fed a high-fat diet. Life Sci 2007;81(17-18):1339-45
- Chen Y, Wu X, Yu S, Neuroprotection of tanshinone IIA against cerebral ischemia/reperfusion injury through inhibition of macrophage migration inhibitory factor in rats. PLoS One 2012;7(6):e40165
- Li YS, Wang ZH, Wang J. Effect of tanshinone II A on angiotensin receptor in hypertrophic myocardium of rats with pressure over-loading. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28(7):632-6
- Yu XY, Lin SG, Zhou ZW, Role of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza Bunge. Curr Drug Metab 2007;8(4):325-40
- Zhang W, He H, Liu J, Pharmacokinetics and atherosclerotic lesions targeting effects of tanshinone IIA discoidal and spherical biomimetic high density lipoproteins. Biomaterials 2013;34(1):306-19
- Tillhon M, Guaman Ortiz LM, Lombardi P, Berberine: new perspectives for old remedies. Biochem Pharmacol 2012;84(10):1260-7
- Zhu B, Zhai Q, Yu B. Tanshinone IIA protects rat primary hepatocytes against carbon tetrachloride toxicity via inhibiting mitochondria permeability transition. Pharm Biol 2010;48(5):484-7
- Xu JK, Hiroshi K, Zheng JJ, Protective effect of tanshinones against liver injury in mice loaded with restraint stress. Yao Xue Xue Bao 2006;41(7):631-5
- Xu W, Ren X, Tang L, Composition containing tanshinone ingredient and HMG-CoA reductase inhibitor and application thereof. CN101596317; 2008
- Liu P, Gu L, Xu S. Medicinal composition for preventing and treating atherosclerosis. CN102125567; 2011
- Chan P, Liu IM, Li YX, Antihypertension induced by tanshinone IIA isolated from the roots of salvia miltiorrhiza. Evid Based Complement Alternat Med 2011;2011:392627
- Li Y, Yang Y, Yu D, The effect of tanshinone IIA upon the TGF-beta1/Smads signaling pathway in hypertrophic myocardium of hypertensive rats. J Huazhong Univ Sci Technolog Med Sci 2009;29(4):476-80
- Su CC. Tanshinone IIA potentiates the efficacy of 5-FU in Colo205 colon cancer cells in vivo through downregulation of P-gp and LC3-II. Exp Ther Med 2012;3(3):555-9
- Wang W, Liu E. Medicinal composition of tanshinone IIA sulfate and ferulic acid and preparation method thereof. CN1806799; 2006