Abstract
Introduction: Despite significant scientific advances over the past 60 years towards the development of a safe, nontoxic and effective radiation countermeasure for the acute radiation syndrome (ARS), no drug has been approved by the US FDA. A radiation countermeasure to protect the population at large from the effects of lethal radiation exposure remains a significant unmet medical need of the US citizenry and, thus, has been recognized as a high priority area by the government.
Area covered: This article reviews relevant publications and patents for recent developments and progress for potential ARS treatments in the area of radiation countermeasures. Emphasis is placed on the advanced development of existing agents since 2011 and new agents identified as radiation countermeasure for ARS during this period.
Expert opinion: A number of promising radiation countermeasures are currently under development, seven of which have received US FDA investigational new drug status for clinical investigation. Four of these agents, CBLB502, Ex-RAD, HemaMax and OrbeShield, are progressing with large animal studies and clinical trials. G-CSF has high potential and well-documented therapeutic effects in countering myelosuppression and may receive full licensing approval by the US FDA in the future.
1. Introduction
Exposures to ionizing radiation, whether they are intended or unintended, are currently an undeniable reality and carry potentially catastrophic health consequences Citation[1]. Therefore, medical preparedness and countermeasures are critical security issues, not only for the individual but also for the nation as well Citation[2]. Current nuclear and radiological threats can be categorized into five groups: i) detonation of a sophisticated nuclear weapon (nuclear bomb); ii) detonation of an improvised nuclear device; iii) use of a radiological dispersal device or dirty bomb; iv) use of a simple radiological device; and v) an attack on a nuclear power plant Citation[3]. The number of individuals who will need care after a large-scale event, such as an improvised nuclear device, will be very high. Models suggest that if a device similar to the bomb detonated over Hiroshima struck a city such as Washington DC, up to 175,000 individuals would require intensive medical care and ∼ 300,000 will require clinical management of radiation-induced myelosuppression Citation[4].
Human acute radiation syndrome (ARS) follows intense, acute radiation doses > 1 Gy of whole-body irradiation or significant partial-body irradiation. The major clinical components of ARS include the hematopoietic (2 – 6 Gy), gastrointestinal (GI; 6 – 8 Gy) and cerebrovascular (> 8 Gy) sub-syndromes Citation[5]. The hematopoietic sub-syndrome is characterized by significant, life-threatening blood cytopenias; the GI syndrome is characterized by massive loss of functional intestinal epithelia, resulting in potential fatal pathological sequelae of fluid and electrolyte imbalances, intestinal bleeding and sepsis. The relevant impact of radiation-induced mortality is dependent on dose, exposure rate and the quality of ionizing radiation involved. These sub-syndromes overlap and are oversimplified, especially at extremely high, intense radiation exposures, when pathologies cut across various organ systems of the body causing net clinical problems that surpass problems associated with any single organ system Citation[6-8]. Individuals who fall into either hematopoietic or GI sub-syndromes, or both, can be effectively managed clinically by appropriate medical interventions (i.e., applications of medical countermeasures) and are more likely to be amenable to countermeasures, unlike those exposed to supra-lethal ionizing radiation (> 8 Gy or higher), which causes clinically unmanageable cerebrovascular syndrome. As a result, the former two sub-syndromes are considered appropriate and relevant clinical targets for the development of novel countering agents.
Radiation countermeasures fall into three broad classes: protectors, mitigators and therapeutics. Radioprotectors are administered before exposure to prevent damage Citation[9]. Radiation mitigators are administered shortly after radiation exposure, before exposure symptoms manifest, to accelerate recovery or repair. Radiation therapeutics or treatments are given after symptoms manifest to stimulate repair or regeneration. Numerous candidate radiation countermeasures (specifically radioprotectants and radiomitigators) have been identified (, ,, and ) and are currently being developed largely for US FDA approval and licensing Citation[10-14]. Some agents have recently been applied for and received patents (). Radioprotectants will be useful for military personnel and first responders, as well as civilians expected to be exposed to fallout fields during evacuation procedures or rescue missions. Although the search for suitable radiation countermeasures was initiated more than half a century ago, no safe and effective radiation countermeasure for ARS has been approved by the US FDA.
Figure 1. Radiation countermeasures under development. Currently, there are seven radiation countermeasures that have US FDA IND status: Androstenediol (5-AED), BIO 300, CBLB502, Ex-RAD, HemaMax, Neupogen and OrbeShield. Neupogen and Leukine are expected to obtain US FDA Emergency Use Authorization and both are available in the SNS. Promising molecules at different stages of development are presented under different groups.
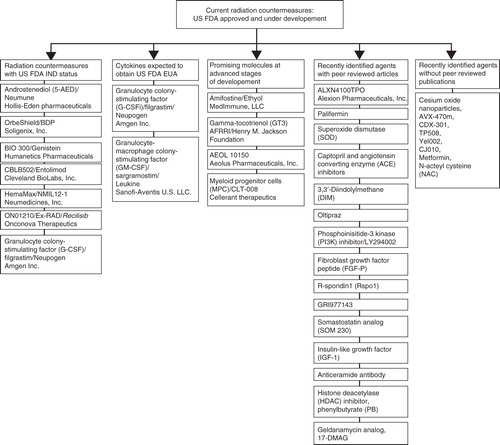
Table 1. Promising radiation countermeasures in advanced stages of development with US FDA IND status*.
Table 2. Radiation countermeasures already in SNS that may receive US FDA EUA status.
Table 3. Other promising radiation countermeasures at advanced stages of development requiring US FDA IND status.
Table 4. Promising radiation countermeasures in the early stages of development.
Table 5. Patents for radiation countermeasures (protectors, mitigators and therapeutics/treatments).
Here, we have reviewed the recent developments of radiation countermeasures for ARS, based on relevant publications and patents. We have placed emphasis on agents in advanced development and agents identified as potential radiation countermeasures since 2011. It is important to note that exact radioprotective mechanisms of action of the majority of these agents under development are complex, involve multiple damage/repair pathways, and are not well understood, but are actively being investigated. However, the majority of radioprotective agents discussed here in this review tend to impact essential cytopoietic feedback loops with given tissues of the body by mimicking the natural ‘lineage-specific regulators’. They act as specific growth regulators tied to secondary feedback loops that ‘sense’ cell content within lineage compartments during steady-state or disequilibrium (cell deficits).
2. Discussion of selected radiation countermeasures under development and new patents
An ideal radiation countermeasure should: i) protect against both acute and chronic radiation damages; ii) be suitable for oral (p.o.) administration with rapid absorption and distribution throughout the body; iii) have no significant toxic side effects including behavioral; iv) be readily available and inexpensive; and v) be chemically stable for easy handling, transport and storage for field use Citation[14]. No single candidate drug has all of these ideal features, although many of the potential radiation countermeasures currently identified have not yet been fully characterized ().
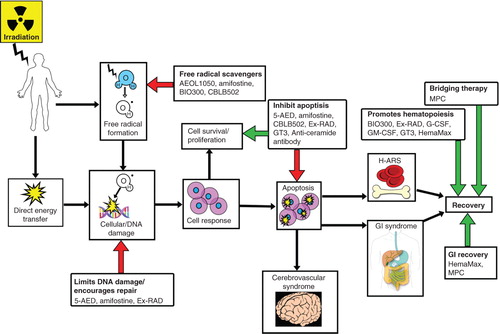
Figure 2. Brief diagrammatic representation of radiation injury and the mode of action of radiation countermeasures at advanced stages of development. This simplified response pathway of a subject’s irradiation shows that radiation exposure induces free radicals, DNA breaks and apoptosis. The various radiation countermeasures reduce the injurious effects of irradiation through different pathways as indicated by colored arrows. Only the drugs with well-understood mechanism of action are included and may have been indicated at multiple points, as several drugs work through several pathways. Red arrows indicate inhibition of deleterious effects of radiation injury and green arrows indicate enhancement of recovery.
2.1 Radiation countermeasures in advanced stages of development with US FDA investigational new drug status
The following seven drugs are currently in advanced development and have been granted US FDA investigational new drug (IND) status: 5-Androstenediol (5-AED)/Neumune, beclomethasone 17,21-dipropionate (BDP)/OrbeShield, BIO 300 (Genistein), CBLB502/Entolimod, HemaMax/NMIL12-1 (recombinant human interleukin-12: rHuIL-12), ON01210/Ex-RAD/Recilisib and filgrastim/Neupogen (G-CSF). Each of these drugs is discussed in the Section immediately below; however, G-CSF has been reviewed in conjunction with GM-CSF in a later Section (2.2), as both have similar mechanisms of action and efficacies, have been used as an off-label ARS treatment and may receive FDA Emergency Use Authorization (EUA). All above countermeasures with FDA IND have a strong possibility for clinical use in the future, but the final outcome will largely depend on the results of clinical trials (safety and toxicity) being conducted with these drugs. Detailed results of such trials with all the agents are not available at this moment.
2.1.1 5-AED/Neumune
5-AED (androst-5-ene-3β,17β-diol) was the first drug to receive US FDA IND status as a radiation countermeasure specifically for treatment and prevention of ARS. It was investigated as both a radioprotector and radiomitigator and was advanced by Hollis-Eden Pharmaceuticals (San Diego, CA, USA) Citation[15-17]. Studies showed a single injection of 5-AED, before or after a lethal dose of total-body irradiation (TBI), enhanced survival in mice; drug administration post-irradiation had a lower efficacy than a pre-irradiation injection Citation[15,18,19]. The radiomitigator efficacy of 5-AED was confirmed with both mice and nonhuman primates (NHPs) Citation[16,20]. 5-AED treatments improved overall blood profiles, including blood platelet levels Citation[15-17,21]. 5-AED significantly increased blood plasma levels of both G-CSF and IL-6 in mice; subsequent ‘neutralization studies’ suggested that G-CSF was, in part, responsible for the drug’s survival enhancement Citation[22]. 5-AED also induced BAX and BCL-2, upregulated CDKN1A and DDB1 (but not GADD45a) expressions and limited DNA strand breakage in splenocytes from irradiated mice Citation[23]. In sum, these results suggest that 5-AED survival enhancement is G-CSF-dependent, and it reduces radiation-induced DNA damage via induction of genes that modulate cell cycle progression and apoptosis. Clinical trials suggest that parenteral (intraperitoneal, i.p.) administration of 5-AED may be a safe and effective means to stimulate innate immunity and alleviate ARS-associated neutropenia and thrombocytopenia Citation[24]. The results of clinical trials are not available in publication.
2.1.2 BDP/OrbeShield
BDP is a highly potent, topically active corticosteroid being developed by Soligenix, Inc. (Princeton, NJ, USA) as a radiation countermeasure for GI syndrome Citation[25]. It provides a potent topical anti-inflammatory effect with less systemic toxicity than a comparably effective systemic corticosteroid. OrbeShield demonstrated a statistically significant survival advantage in a canine model of GI sub-syndrome Citation[25]. Canines received TBI, followed by autologous bone marrow infusion and supportive care. The experiment had three groups: one control group (no drug given) and two treatment groups with varying treatment schedules. Both BDP-treated groups had significantly enhanced survival compared with the control group Citation[26]. These findings suggest that BDP has the potential to rescue inflamed tissues in the radiation-damaged GI mucosa and improve survival when therapy is initiated as late as 24 h after high-dose irradiation, which is relevant to the problem of identifying and developing appropriate medical countermeasures for the GI sub-syndrome. The FDA has granted IND status, orphan drug and fast-track statuses to OrbeShield, which has been formulated as a single product consisting of two tablets for p.o. administration to patients with GI ARS; one tablet is intended to release BDP in the proximal and the other in the distal portions of the GI tract Citation[25]. There is no report regarding clinical trial with this agent yet. BDP has been marketed in the US as the active pharmaceutical ingredient in a nasal spray and in a metered-dose inhaler for the treatment of allergic rhinitis and asthma.
2.1.3 BIO 300
Isoflavone (4′,5,7-trihydroxyisoflavone), known as genistein, is a phytoestrogen, antioxidant (free-radical scavenger) and protein tyrosine kinase inhibitor that modulates signal transduction pathways and is being developed by Humanetics Pharmaceuticals (Minneapolis, MN, USA) under the name BIO 300 Citation[13,27,28].
The FDA has granted orphan drug designation and IND status to BIO 300 for the prevention of ARS. Genistein protects mice against the potential lethal effects of 60Co γ-radiation when administered before TBI Citation[29,30]. The dose reduction factor (DRF) for genistein is 1.16 when administered subcutaneous (sc). Multiple oral doses of genistein significantly protected mice against γ-irradiation Citation[31,32]. When administered before irradiation, genistein reduces lung injury in mice Citation[33]. Genistein stimulated induction of low levels of hematopoietic cytokines Citation[34]. A single sc or intramuscular injection of genistein before irradiation provided significant radioprotection to the hematopoietic system of the exposed animal, including both the proliferating/differential myeloid elements, as well as the progenitorial marrow compartments Citation[31,35]. Pretreatment with sc genistein appears to limit radiation-induced senescence of primitive hematopoietic tissue repopulating progenitors,that is, LSK (lineage- Sca-1+ c-kit+) cells Citation[36]. LKS progenitors from genistein-treated mice expressed fewer DNA damage-responsive and cell-cycle checkpoint genes than did LSK cells from untreated or vehicle-treated mice. Interestingly, a combination of sc injected genistein and orally administered captopril (ACE inhibitor, a vasodilation drug) increased the radioprotective efficacy of genistein in C57Bl/6J mice Citation[37].
The effectiveness of p.o. genistein is limited by relatively poor bioavailability. However, nanoparticle formulations have produced an easy-to-use p.o. or intramuscular (i.m.) preparation that can be taken without medical supervision Citation[38]. The nanoparticle formulation injected i.m. was shown to afford mouse protection, increase bone marrow cellularity and decrease radiation-induced death of hematopoietic stem and progenitor cells Citation[39]. Humanetics Pharmaceuticals has conducted a Phase I clinical trial and reported that BIO 300 is safe and well tolerated when administered p.o. for 14 days in healthy volunteers Citation[38].
2.1.4 CBLB502/Entolimod
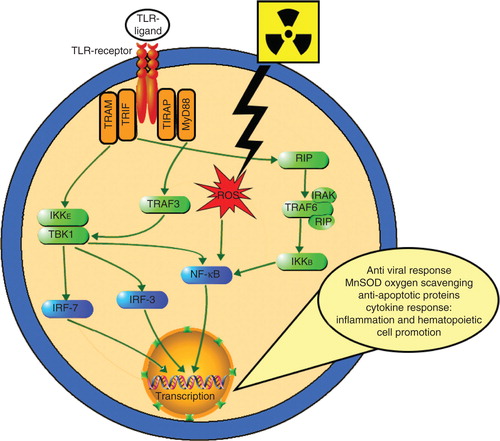
CBLB502, a truncated derivative of the Salmonella bacteria (Salmonella enterica serovar Dublin) flagellin protein, is a potent and stable agent, currently being clinically developed as a radiation countermeasure by Cleveland BioLabs, Inc. (Buffalo, NY, USA) Citation[40]. Its pharmacologic action is based on its binding to toll-like receptor 5 (TLR5) of targeted cells and activating NF-κB signaling (), which modulates expression of numerous genes, including inhibitors of apoptosis, scavengers of reactive oxygen species and a spectrum of cytokines. Cleveland BioLabs, Inc. identified CBLB502 as a TLR5 ligand that significantly improved the radioprotective efficacy of native flagellin, while having significantly reduced toxicity and immunogenicity Citation[41].
Figure 3. Schematic representation of TLR-ligand-mediated NF-κB activation. TLR ligands (multiple, not all radioprotective) interact with TLR receptor inducing two divergent signaling pathways controlled by two pairs of adaptor proteins: TRAM/TRIF and TIRAP/MyD88. The MyD88-dependent pathway quickly upregulates inflammatory cytokines via NF-κB activation by its dissociation from inhibitory component (IκB). This permits NF-κB to enter the nucleus where it can ‘turn on’ the expression of specific genes such as inflammatory or immune response, a cell survival response, cellular proliferation and oxygen-scavenging MnSOD. The MyD88-independent pathway does this as well, in addition to inducing type-1 IFN expression through IRFs and triggering IFN-β, which results in cell maturation. MyD88-independent pathways are activated with slower kinetics. Radiation produces ROS, which also activate NF-γB.
Purified flagellin protects mice from lethal doses of γ-TBI Citation[42]. A single injection of CBLB502 between 24 h before and 48 h after lethal TBI protected mice from both GI and hematopoietic sub-syndromes with significantly improved survival Citation[43]. Similar radioprotective and radiomitigative potentials are documented in lethally irradiated NHPs Citation[41].
Two cytokines, G-CSF and IL-6, were identified as candidate biomarkers for CBLB502’s radioprotective and radiomitigative efficacies and were found to be crucial for CBLB502’s ability to increase survival of irradiated animals; administration of either G-CSF or IL-6 neutralizing antibody abrogated its protection in acutely irradiated rodents Citation[44]. These biomarkers will likely be useful in accurately predicting the radioprotective or radiomitigative dose of CBLB502 in humans. Like other radiation countermeasures, the FDA has granted IND, fast-track and orphan drug statuses to CBLB502 as a radiation countermeasure. It is currently in clinical development, and a human safety study indicated that CBLB502 was systemically well tolerated and had biomarker results corresponding to previous biomarkers data from animal ARS models Citation[44,45]. Cleveland BioLabs, Inc. is preparing EUA application for submission to the FDA.
2.1.5 HemaMax/NMIL12-1
HemaMax is rhuIL-12, a heterodimeric cytokine shown to play an important role in regulating inflammatory responses, stimulating IFN-γ production from natural killer (NK) cells, macrophages and T cells Citation[46,47]. Mouse-specific IL-12 demonstrated increased mice survival when a single dose was administered either 24 h before or within 1 h after TBI exposure Citation[48,49]. Currently, rhuIL-12 is being developed as a radiomitigator by Neumedicines, Inc. (Pasadena, CA, USA). The pharmacokinetics, pharmacodynamics and efficacy of mouse and human IL-12 in mice and rhesus NHP (Macaca mulatta), respectively, have been reported Citation[50]. Allometrically equivalent doses of Mouse HemaMax and (human) HemaMax preparations significantly increased mouse and NHP survival, respectively, when administered 24 h post-irradiation with similar pharmacokinetics. In the NHP study, survival benefit was accompanied by a higher leukocyte, thrombocyte and reticulocyte counts during nadir (12 – 14 days) and less body weight loss when compared to vehicle. These results provided ‘proof of concept’ that HemaMax increases survival in irradiated NHPs by promoting hematopoiesis and recovery of immune function and GI function. Recently, another group confirmed the radiomitigation potential of rhuIL-12 in NHPs Citation[51]. Murine HemaMax has also been evaluated in the mouse model of combined injury (radiation and wound). Topical administration after radiation exposure has been demonstrated to enhance wound closure suggesting that it could serve as a multifunctional mitigator of combined injuries Citation[52].
Neumedicines reported a significant increase in survival, when NHPs were treated with a single, low dose of rhuIL-12. This study had additional treatment groups that received G-CSF for 18 consecutive days and another that received G-CSF for 18 consecutive days in combination with a single dose of rhuIL-12. No antibiotics, fluids or blood products were administered. Single rhuIL-12 treatments significantly decreased frequency of severe neutropenia (< 100 cells/μl) and severe thrombocytopenia (< 10,000 cells/μl) when compared to vehicle or G-CSF treatment groups. The combination of G-CSF plus rhuIL-12 appeared to augment the recovery of trilineal hematopoiesis to a greater extent than with just rhuIL-12; however, this did not translate to improved survival. These data demonstrate that G-CSF can be safely administered after rhuIL-12. However, it needs to be noted that essential elements of study needed for comparisons (drug dose, radiation doses and animal monitoring periods) are not available on Neumedicine’s website Citation[46]. Additionally, these results conflict with those of a recent study that demonstrated, for the first time, a benefit for G-CSF administered post-irradiation along with an intensive, trigger-based medical management regimen Citation[53].
To demonstrate the safety of HemaMax, Neumedicines conducted a Phase Ib study where healthy human volunteers were administered a single dose predicted to be effective in humans for treating hematopoietic syndrome based on NHP data; this trial suggests rhuIL-12 to be safe and well tolerated at this dose Citation[46]. An additional study investigating the mechanism of action of rhuIL-12 in healthy human subjects suggests that rhuIL-12 administration induced IL-12Rβ2+, CD16+CD56+ NK cell migration from the peripheral blood into the tissue compartment, through a mechanism facilitated by IFN-γ-induced CXCL10 chemokine and its receptor CXCR3 Citation[46].
The above studies suggest that rhuIL-12 has potential to be an effective radiation mitigator against radiation lethality. Neumedicines is advancing the development of rhuIL-12 towards a Biologic License Application submission to the FDA under the Animal Efficacy Rule for the treatment of the hematopoietic sub-syndrome of ARS Citation[54].
2.1.6 ON01210/Ex-RAD/Recilisib
ON01210 (a chlorobenzyl sulfone derivative) is a novel, small-molecule kinase inhibitor in development as a radiation countermeasure by Onconova Therapeutics (Newtown, PA, USA), also known as Ex-RAD/Recilisib. Unlike most radioprotectors, Ex-RAD is not a free-radical scavenger or responsible for cell cycle arrest. Available data suggest that Ex-RAD has a novel mechanism for radiation protection involving DNA repair pathways Citation[55,56]. Ex-RAD provided significant protection against 60Co γ-irradiation when administered to mice before radiation exposure sc or p.o. Citation[57]. Ex-RAD’s estimated DRF is 1.16 (sc, prophylactic) Citation[55]. Ex-RAD’s radiomitigative properties have been explored as well but less extensively than its radioprotective properties. Ex-RAD provided mitigation to acutely irradiated mice when the drug was administered after radiation exposure Citation[58]. To examine Ex-RAD’s efficacy, additional studies are needed using higher, whole-body radiation doses. Ex-RAD clearly protects the hematopoietic system, increasing survival of irradiated mice; administration quickly alleviates the severe, radiation-induced pancytopenia and restores selected marrow functions Citation[57,59]. Mechanistically, Ex-RAD appears to protect both marrow cells and intestinal crypt cells from radiation-induced apoptosis Citation[59]. These protective actions may be due, in part, to signaling pathways that are affected by Ex-RAD. Attenuation of ATM-p53-mediated DNA damage response (DDR) by Ex-RAD likely contributes to the mitigation of radiation-induced hematopoietic toxicity Citation[56]. Further, Ex-RAD appears to function through the upregulation of PI3-kinase/AKT pathways in cells exposed to radiation Citation[60].
The Defense Medical Research and Development Program (US Department of Defense), and Biomedical Advanced Research Development Authority (BARDA) (Health and Human Services) have funded research to study the efficacy and identify biomarkers in the NHP animal. These studies will be conducted at the Armed Forces Radiobiology Research Institute, Bethesda, MD, USA.
Onconova Therapeutics has completed four Phase I clinical studies using Ex-RAD in healthy volunteers. Three of these trials administered Ex-RAD sc and one, p.o.; none of these trials reported evidence of systemic side effects Citation[61]. Oral administration holds better clinical promise as an effective countermeasure for first responders as well as for at-risk civilian populations in a nuclear accident. Among promising radiation countermeasures with US FDA IND, only Ex-RAD, OrbeShield and BIO 300 have demonstrated efficacy when administered through the p.o. route. However, due to BIO 300’s multiple dosing requirement for efficacy, this drug is somewhat less attractive than Ex-RAD (two doses) for development as a general medical countermeasure for ARS.
2.2 Cytokines that may be approved by US FDA for EUA – already used off-label for radiation accident victims
The two growth factors G-CSF and GM-CSF may receive full licensing approval by the FDA. Neither is FDA approved for treating ARS; however, both growth factors are currently included in the Strategic National Stockpile Citation[62]. As stated earlier, G-CSF, but not GM-CSF, has FDA IND status Citation[63].
2.2.1 G-CSF
The radioprotective efficacy of G-CSF has been evaluated in different strains of mice Citation[64-67], canines (beagle) Citation[68-72], NHPs Citation[53] and recently, minipigs Citation[73]. Because G-CSF is not species-specific, a majority of these studies have used human recombinant G-CSF (Neupogen/filgrastim, Amgen, Inc., Thousand Oaks, CA, USA), although some used the pegylated form of G-CSF (Neulasta/pegfilgrastim, Amgen, Inc.) or G-CSF from other sources. The results of these numerous studies suggest that G-CSF consistently enhanced survival and blood leukocytes (neutrophils) recovery across species (mice, canine, minipig and NHP) regardless of radiation source (γ-ray, X-ray). G-CSF has also been shown to be an effective mitigator of injury against mixed field irradiation (neutron and γ-photon) in mice Citation[74]. The radioprotective efficacy of G-CSF is dependent not only on the extent of radiation exposure but also on the drug dose, treatment schedule in relation to radiation exposure and duration of the treatment. The Centers for Disease Control and Prevention currently has an IND application (with the US FDA) containing a clinical protocol for how G-CSF/filgrastim would be administered to exposed victims in the event of a radiological nuclear incident Citation[63].
Neulasta and Maxy-G34 are pegylated versions of native G-CSF that have well-documented radioprotective, survival-promoting efficacies against potential lethal radiation exposures of both mice and NHPs Citation[75]. Studies clearly demonstrated that pegfilgrastim can be administered in very limited fashion and still retain the therapeutic benefits of more extensive dosing regimens required by filgrastim, for example, 2 weekly injections of pegfilgrastim are equivalent or significantly better in virtually all measured parameters that reflect granulopoiesis compared to 17 – 21 days of daily filgrastim injections Citation[76].
An additional study demonstrated that while pegylated G-CSF limits the severity of the radiation-induced cytopenias in an ARS rodent model, this agent appears to be less efficacious in treating irradiated animals with substantial skin burns (15% total body surface area skin burns) Citation[77]. Although G-CSF (filgrastim) has not been tested in the murine model of irradiation and burn, G-CSF appears to protect both irradiated and combined injury (irradiation and wounded) mice.
2.2.2 GM-CSF
The radioprotective efficacy of GM-CSF (sargramostim/Leukine) has been evaluated in mice, canines and NHPs Citation[68,78,79]. Unlike G-CSF, GM-CSF is species-specific. rhuGM-CSF and recombinant canine GM-CSF have been used in NHP and canine studies, respectively. Sargramostim can be used instead of recombinant mouse GM-CSF for murine studies.
GM-CSF enhances the recovery of blood leukocyte levels in various strains of mice Citation[78,80-82], canines Citation[68,83,84] and NHPs Citation[79,85,86] when administered alone or in combination with other cytokines. Like G-CSF, GM-CSF administration decreased the severity and duration of neutropenia, enhanced neutrophil recovery, along with overall recovery of blood leukocyte counts, and increased granulocyte-macrophage colony-forming units in the bone marrow. However, survival benefits of GM-CSF treatment are inconsistent between studies; the causes of inconsistency could not be definitively identified because of the dissimilarities in preparation, sources of GM-CSF and the various study designs. Results available in the published literature consistently support recovery from severe neutropenia as a benefit of using GM-CSF in hematopoietic ARS. In mice and canine studies where the efficacies of G-CSF and GM-CSF have been compared side by side, G-CSF was found to be more effective in protecting irradiated animals Citation[69,81].
2.3 Other promising molecules at advanced stages of development
Additional radiation countermeasures in advanced stages of development include: amifostine, γ-tocotrienol and myeloid progenitors.
2.3.1 Amifostine/Ethyol
Amifostine (WR2721, 2-(3-aminopropyl) aminoethylphosphorothioate), is the only systemically effective radioprotective agent that has been fully approved (June 1999) for human use by the US FDA Citation[87-90]. Despite the FDA’s approval, the drug has only been authorized for use for a very narrowly defined medical indication, namely the reduction of xerostomia (dry mouth) that results from injury of salivary glands in patients undergoing radiotherapy for the treatment of head and neck cancers Citation[91]. The secondary indication for amifostine’s use (and approval) is for the cytoprotection of irradiated oral epithelium and the prevention of oral mucositis. Despite this very limited drug indication, amifostine is well recognized as a potent cytoprotectant for many of the body’s major organ systems when administered at sufficiently large doses (i.e., 300 – 500 mg/kg). In rodent models of ARS and depending on the dose of amifostine administered, DRFs have been estimated to range from 1.6 to 3.0 for hematopoietic and from 1.6 to 2.1 for GI syndrome Citation[87]. In general, the drug doses cited above are well within the range of doses currently prescribed (∼ 200 mg/m2/d delivered slowly by intravenous [i.v.] infusions or ∼ 500 mg/kg/d delivered slowly by sc injection). Under standard dosing regimens, amifostine is generally well tolerated by the majority of patients; serious side effects are rare; however, minor toxic responses occur frequently which include nausea, vomiting and adverse cutaneous reactions following sc injection. It is because of these performance-decrementing side effects that amifostine has not been approved for general use in radiation protection of high-risk personnel or the population at large. Alternative indications have been proposed for amifostine including: i) global cytoprotection with significant survival benefit when administered at high drug doses, notwithstanding the potential risks of toxic side effects; ii) selective protection of specific progenitorial tissue compartments at low drug doses Citation[92]; and iii) protection against late-arising, radiation induced cancers with low drug doses Citation[14].
In addition to the side effects, there are other limitations in using this drug for nonclinical purposes. First, amifostine is currently administered by i.v. infusion; other routes of drug delivery have been explored but remain to be authorized by the FDA. Second, amifostine has an extremely short, pre-exposure time-window of radioprotectiveness (i.e., generally < 1 h).
Despite amifostine’s limitations, it is hard to dismiss the drug as a viable radioprotectant for nonclinical applications; amifostine is a remarkably effective, potent and systemically active drug that has the capacity not only to provide substantial cytoprotection to various vital bodily tissues, but also to promote survival in otherwise fatal nuclear/radiological exposure situations. Notwithstanding the potential toxic side effects of the drug, amifostine might be effectively administered (or self-administered) to general populations who are not tasked to perform essential emergency tasks (e.g., at risk, general resident populations being asked to ‘shelter-in-place’).
It was logical to test whether amifostine could enhance the radioprotective efficacy of another drug that acts through a different pathway, such as γ-tocotrienol (GT3). Amifostine (doses of 30 and 50 mg/kg) enhanced efficacy of a significantly low dose of GT3 (50 mg/kg, optimal dose of GT3 is 200 mg/kg in mice). This study suggests that both agents can be used at lower doses and still achieve optimal radioprotection against a lethal dose of radiation without producing adverse effects (Vijay K. Singh – unpublished observation).
Despite the modest advancements in maximizing amifostine’s radioprotective utility, none of the strategies to make use of amifostine have entirely eliminated the problem of amifostine’s toxicity. Although the work on amifostine and related aminothiols has been promising in terms of developing and fielding a safe and effective radioprotector, additional research is clearly needed in order to improve current drug design and delivery strategies.
2.3.2 γ-Tocotrienol
GT3 is one of the eight isomers (tocols) of vitamin E Citation[93]. It is a potent inhibitor of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase Citation[94,95]. In recent years, GT3 appears to be one of the more promising radioprotective tocols tested to date. GT3 has been shown to increase survival in rodents, through ameliorating the radiation-induced injuries of the hematopoietic and GI systems Citation[96,97]. When administered sc 24 h before irradiation, GT3 significantly protected mice against radiation and had an estimated DRF of 1.29 based on survival of CD2F1 mice. GT3 treatment accelerated hematopoietic recovery, improved peripheral blood profiles, enhanced recovery of hematopoietic progenitors in marrow of irradiated mice and induced relatively high levels of G-CSF and IL-6 in mice Citation[98-100]. Studies suggested the most efficacious time for GT3 administration is 24 h prior to irradiation, possibly due to the induction of key hematopoietic cytokines. Prophylactic GT3 administration upregulates anti-apoptotic genes and downregulates pro-apoptotic genes Citation[101]. Jejunal crypt analysis showed protection of GI tissue by GT3 treatment. The protective action of GT3 can be abrogated by G-CSF-specific antibody Citation[102].
The radioprotective efficacy of GT3 has been tested at two drug dosing levels without supportive care in a NHP model of radiation-induced ARS. Three different radiation doses were used in this study. Results demonstrate that the GT3 treatments significantly decreased the duration and the severity of neutropenia and thrombocytopenia (Vijay K. Singh – unpublished observation). It is important to note that GT3 when administered in one dose, was comparable to results achieved using multiple G-CSF administrations in combination with supportive care, in terms of improving the radiation-induced neutropenia and thrombocytopenia. Another isomer of vitamin E, delta-tocotrienol, has demonstrated comparable radioprotective efficacy in the murine model Citation[103-107].
2.3.3 AEOL 10150
AEOL 10150 (meso-porphyrin mimetic; C48H56C15MnN12) is a radiation countermeasure being developed by Aeolus Pharmaceuticals, Inc. (Mission Viejo, CA, USA). AEOL 10150 is a novel, well-tolerated (as evidenced in initial clinical trials) antioxidant with significant protective and mitigative potential relative to acute radiation injuries, particularly acute pulmonary injury Citation[108-112]. Extended survival and minimization of acute pathology has been demonstrated using sustained, drug dosing regimens of AEOL 10150 (e.g., daily treatments for 28 days) using both murine and NHP models Citation[113]. Garofalo et al. investigated whether administration of AEOL 10150 after thoracic exposures in NHPs could reduce radiation-induced lung injury and improve overall survival Citation[114]. Results indicated that daily drug administrations, beginning after irradiation, effectively mitigated potentially fatal radiation-induced lung injury and improved survival. Plasma analysis suggested that AEOL 10150 treatment led to lower TGF-β1 levels. AEOL 10150 treatments resulted in significant reductions of radiation-induced lung injury, as evidenced by reduced clinically, radiographically, anatomically and by molecular markers of injury.
This agent is also being investigated as a countermeasure for GI and, if successful, it may be exceedingly useful for treating multiple sub-syndromes of ARS that occur following very high radiation exposures, which is difficult to manage clinically Citation[111]. The drug captures and neutralizes reactive oxygen and nitrogen species generated by ionizing radiation, in turn reducing oxidative stress, inflammation and signals in injury-eliciting tissue damage signaling cascades.
2.3.4 Myeloid progenitor cells
Myeloid progenitor cell (MPC) are being developed as a cellular bridging therapy, administered after radiation exposure, to provide hematopoietic cellular support, allowing bone marrow stem cells to repopulate the blood system. Administration of pooled, cryopreserved allogeneic mouse MPC (mMPC) significantly improved 30-day survival of lethally irradiated recipient mice Citation[115]. mMPC transfusion appears to mitigate multi-organ failure occurring at extremely high doses of irradiation; the DRF of 5 million mMPC administered 24 h post-irradiation to CD2F1 mice is 1.73. Jejunum histopathology of irradiated and mMPC-transfused mice revealed improved structural integrity; the infusion of mMPC significantly decreased the number of bacterial infections and lowered endotoxin levels in serum Citation[116]. These studies support the contention that the transfusion of MPC acts as a bridging therapy for both the hematopoietic system and GI system.
The following additional characteristics make MPC a potentially ideal treatment for sizable numbers of nuclear/radiological exposure accident victims: i) MPCs can be cryopreserved and stored without compromise in function and ii) treatment can be delayed up to 7 days and still provide survival benefit. However, promising, additional studies are required to identify cell populations differentiated from myeloid progenitors and rule out graft versus host disease. These preclinical studies need to be transitioned to work using large animal models, to provide long-term follow-up of recipients receiving different radiation doses and to study the clearance of donor cells from recipients which may depend on the extent of their radiation injury.
Cellerant Therapeutics, Inc. (San Carlos, CA, USA) is developing CLT-008, a unique, cell-based therapy containing human myeloid progenitors as a treatment for chemotherapy-induced neutropenia and as an adjunct to cord blood transplantation Citation[117]. Derived from adult hematopoietic stem cells, it has the ability to mature into functional granulocytes, platelets and red blood cells in vivo and is currently in a Phase I study. Cellerant Therapeutics also has product CLT-009 under development for treatment of thrombocytopenia.
2.4 Agents identified recently and under early stages of development
Although large numbers of agents have been identified recently and are currently under development, we have selected only those agents that have animal survival data published in peer-reviewed papers. Additional agents presented during conferences but lacking peer-reviewed publications such as cesium oxide nanoparticles, AVX-470m (a murine TNF-specific surrogate of AVX-470, a novel polyclonal anti-TNF antibody), CDX-301, TP508, Yel002 and CJ010 (analog of Yel002), metformin and N-acetyl cysteine have not been included in this article.
2.4.1 ALXN4100TPO
ALXN4100TPO is a novel thrombopoietin (TPO) receptor agonist, synthesized by Alexion Pharmaceuticals, Inc. (Cheshire, CT, USA) to reduce the potential of endogenous generation of TPO antibody. With no sequence homology to the native TPO glycoprotein, ALXN4100TPO’s binding kinetics to TPO’s receptor is equipotent to the native molecule, as demonstrated in an in vitro receptor assay. The synthetic agonist has been shown to stimulate megakaryopoiesis in two different mouse strains and effectively prevents radiation-induced lethality in mice by abrogating thrombocytopenia and bone marrow atrophy Citation[118]. Estimated DRFs in irradiated mice are 1.32 when administered prophylactically and 1.11 when delivered 12 h post-exposure Citation[114]. Reports indicate that ALXN4100TPO administered sc results in stimulated extramedullary hematopoiesis, with no immediate, life-threatening adverse health effects Citation[119]. Although ALXN 4100TPO has some efficacy as a radiation countermeasure against γ-radiation, it did not provide protection against mixed field γ and neutron radiations Citation[74]. The agent’s potential for adverse, long-term health effects need to be thoroughly evaluated.
2.4.2 Palifermin
Palifermin is a recombinant N-terminal truncated form of keratinocyte growth factor (KGF), also known as fibroblast growth factor 7 (FGF7). KGF, produced by mesenchymal cells to protect and repair epithelial tissues, acts on its targets through the FGFR2B receptor. KGF significantly promotes the recovery of mucosa from radiation-induced injury Citation[120]. KGF also improves epithelial barrier functions after irradiation and limits bacterial translocation and subsequent sepsis (Seed et al. – unpublished observations). Palifermin-protective action appears to be due to a combination of proliferation stimulation and anti-apoptotic actions Citation[121]. Preclinical studies suggest that palifermin ameliorates mucosal toxicity of chemotherapy and/or radiation therapy Citation[121]. Palifermin reduces the incidence, duration and severity of oral mucositis in chemoradiotherapy patients with head and neck cancers as well as patients receiving other chemotherapy agents causing mucositis. Palifermin has also mitigated esophagitis-induced dysphagia in patients treated with chemoradiotherapy for lung carcinoma. Interestingly, palifermin also appears to stimulate immune reconstitution following hematopoietic stem cell transplantation and to reduce graft versus host disease following allogeneic bone marrow transplantation Citation[122].
2.4.3 Superoxide dismutase
Over-expression of manganese superoxide dismutase (MnSOD, also called SOD2) by injection of a replication-deficient adenovirus containing the MnSOD transgene conferred protection against lung irradiation and cytokine production (IL-1, TNF-α and TGF-β) when administered prior to irradiation Citation[123]. MnSOD appears to protect against radiation-induced apoptosis in cultured cell lines, in part, by stabilizing the mitochondrial membrane Citation[124]. In a mouse model of radiation-induced oral mucositis, p.o. administration of MnSOD decreased radiation-induced ulceration Citation[125]. These findings suggest the possibility of utilizing radioprotective antioxidant gene therapy to prevent or reduce the extent of some forms of radiation injury. Following 9.5 Gy TBI, MnSOD-treated mice that developed and recovered from ARS and fed a diet rich in antioxidants showed an increased lifespan when compared to comparably treated and ARS-recovered mice that had been fed only the standard house diet Citation[126]. These findings suggest that when combined with MnSOD treatments, enriched antioxidant/chemopreventive diets have the potential to increase the lifespans of ARS survivors.
2.4.4 Captopril and ACE inhibitors
Captopril, a competitive inhibitor of ACE protease, was initially developed for hypertension and heart failure treatment; later, it was found to increase renal plasma flow and improve glomerular filtration in animal models of radiation-induced renal dysfunction Citation[127,128]. Captopril has been investigated as a radiation countermeasure for the pulmonary, renal and hematopoietic systems as well as brain and skin Citation[129-134]. ACE inhibitors and captopril mitigated radiation-induced pulmonary endothelial dysfunction, radiation pneumonitis and fibrosis in animal models Citation[135,136]. Prophylactic administration of captopril lowered systemic blood pressure and improved renal function following TBI in animal models and reduced chronic renal failure in human radiation therapy patients Citation[128,137-139]. Additionally, captopril and perindopril, another ACE inhibitor, were demonstrated to limit radiation-induced cytopenias through maintenance and recovery of blood cell levels, which has been reported as specifically associated with protection and regrowth of selected hematopoietic progenitor populations Citation[129,140]. These observations need to be confirmed in large animal models of radiation-induced ARS. The mechanism(s) of captopril-induced reduction of radiation injury has not been firmly established, but it is believed to be related to thiol-mediated ‘free-radical scavenging.’
2.4.5 3,3′-Diindolylmethane
3,3′-Diindolylmethane (DIM) is a small molecule compound formed when indole-3-carbinol (I3C) is hydrolyzed in the stomach Citation[141]. DIM is a proposed ‘cancer prevention’ nutritional supplement and has been administered safely to humans in repeated oral doses in Phase I/II clinical trials Citation[142-145]. A multi-dose schedule of DIM, initiated before or after irradiation, protected rodents against lethal doses of TBI Citation[146]. When i.p. DIM treatment was started 24 h after irradiation, the estimated DRF was 1.6. This work needs to be confirmed, using an appropriate large animal model. Submicromolar DIM concentrations protected cultured cells against radiation by a novel mechanism; DIM caused rapid activation of Ataxia telangiectasia mutated (ATM) and phosphorylation of various ATM substrates, suggesting that DIM induces an ATM-dependent DDR-like response, enhances radiation-induced ATM signaling and NF-κB activation. Similarly, DIM caused ATM activation and signaling in normal tissues in rodents.
2.4.6 Oltipraz
Oltipraz (a synthetic dithiolethione derived from broccoli) administered prior to γ-TBI, increased mouse survival, decreased radiation-induced lipid peroxidation and acid phosphatase, decreased radiation induced inhibition of glutathione and alkaline phosphatase, as well as reduced chromosomal aberration and micronuclei formation Citation[147]. Although the authors reported improvement in GI-related parameters, it is unlikely that a dose of 8 Gy (LD50/30 reported as 6.6 Gy) would have induced substantial damage in GI. An earlier report appears to support these findings concerning Oltipraz’s improved irradiated mice survival at mid-lethal doses but failed to show any significant sparing effect on hematopoiesis. This interesting finding might well suggest that the drug’s protective effects are limited to and associated with enhanced expression of microsomal epoxide hydrolase and glutathione S-transferase genes Citation[148]. Because of the hematological anomalies noted in these reports, additional preclinical work is needed.
2.4.7 Phosphoinisitide-3 kinase inhibitor (LY294002)
Ionizing radiation induces genotoxic stress that triggers adaptive cellular responses, such as activation of the phosphoinisitide-3 kinase (PI3K)/Akt signaling cascade. A single dose of LY294002 (CID3973) or PX-867 (CID24798773) administered after a lethal dose of γ-irradiation significantly enhanced mouse survival Citation[149]. Cell cycle checkpoints are important regulators of cell survival after radiation exposure; cell cycles after γ-irradiation and PI3K inhibitor treatment was investigated Citation[149]. LY294002 and PX-867 treatment decreased the proportion of S phase cells and increased the G1 population. Post-radiation LY294002 and PX-867 treatment also increased the G1 and G2 populations and decreased S phase and DNA damage as measured by γ-H2AX Citation[149]. These results indicate pharmacologic inhibition of PI3K after irradiation abrogates cell death. These observations suggest that rational drug design, based on specific pharmacological ‘targeting’ for radiomitigation need to be more actively and aggressively pursued.
2.4.8 FGF-peptide, a dimerized peptide derived from FGF2
Various members of the FGF family have been reported to mitigate radiation-induced damage Citation[150,151]. FGF-peptide (FGF-P) (synthetic binding domain peptide of FGF-2 with a peptidase resistant dimer form) attenuates both sepsis and bleeding in a radiation-induced hematopoietic syndrome model and reduces the severity of GI and cutaneous syndromes Citation[152]. FGF-P induces little or no deleterious inflammation or vascular leakage, distinguishing it from other growth factors, angiogenic factors and cytokines. Although recombinant FGFs have proven safe in several ongoing clinical trials, they are expensive to synthesize, can only be produced in limited quantity and have limited shelf life. FGF-P has the positive features without the disadvantages. One study shows FGF-P to be a potent, safe, broad-spectrum radiation mitigator, showing promise for thermal burns, ischemic wound healing, tissue engineering and stem-cell regeneration. FGF-P stimulated the growth of bone marrow cells harvested from irradiated mice; the number of leukocytes, granulocytes, pro-B and pre-B cells increased with treatment. In vivo FGF-P treatment increased the long-term hematopoietic stem cells in bone marrow Citation[153]. These data reveal the underlying cellular basis by which FGF-P rescued a significant percentage of the exposed mice.
2.4.9 R-spondin1
Human R-spondin1 (Rspo1), a 29 kDa, 263 amino acid protein, acts as a mitogenic factor for intestinal stem cells; therefore, it was hypothesized that its systemic administration would amplify intestinal crypt cells, accelerate regeneration of irradiated intestine and ameliorate radiation-induced GI syndrome. Mice receiving recombinant adenovirus expressing human Rspo1 (a potent Wnt signal enhancer and one of the four analogs of R-spondin) before potentially lethal TBI or local abdominal irradiation had higher survival than the control group. Histological analysis demonstrated significant structural regeneration of the intestine in treated and irradiated animals. Immunohistochemical analysis demonstrated an increase in Lgr5+ve crypt cells, translocation of β-catenin from the cytosol to nucleus and upregulation of β-catenin target genes in treated mice. The mechanism was likely related to induction of the Wnt-β-catenin pathway and promotion of intestinal stem cell regeneration Citation[154].
2.4.10 2-((3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propyl)thio)benzoic acid (LPA2, GRI977143)
Recently, prototypic non-lipid agonist, GRI977143, has been reported to rescue in vitro and in vivo cells from high-dose γ-irradiation-induced apoptosis. GRI977143 is effective in rescuing mice from lethal irradiation when administered after TBI Citation[155]. This study suggests that by specifically activating lipid mediator lysophosphatidic acid receptor subtype (LPA2), GRI977143 activates the ERK1/2 pro-survival pathway, effectively reducing BAX translocation to the mitochondrion, attenuating the activation of initiator and effector caspases, reducing DNA fragmentation and inhibiting poly (ADP-ribose) polymerase-1 (PARP-1) cleavage associated with γ-irradiation-induced apoptosis. GRI977143 also inhibits bystander apoptosis elicited by soluble pro-apoptotic mediators produced by irradiated cells.
2.4.11 Somatostatin analog (SOM230)
The somatostatin analog, SOM230, has potent radioprotective and radiomitigative effects on GI tissues, which appeared unrelated to a direct cytoprotective effect by the agent. The indirect pathway appears to involve suppression of secretion by GI lumen-modifying pancreatic enzymes. Mice administered SOM230 starting before or after TBI exposures survived at higher rates and exhibited extended survival times regardless of when drug was initially administered Citation[156]; there was no additional benefit when the drug treatments were extended. SOM230 survival benefit was reversed by coadministration of pancreatic enzymes. Consistent with the presumed non-cytoprotective mechanism of action, SOM230 did not influence hematopoietic injury or intestinal crypt lethality; however, SOM230 preserved mucosal surface area and reduced bacterial translocation in a dose-dependent manner. Circulating IL-12 levels were reduced in SOM230-treated mice. Rigorous assessments showed no observed SOM230 toxicity. SOM230 is an excellent radiation mitigator with a post-irradiation time window in excess of 48 h Citation[157]. The mechanism involves decreased secretion of pancreatic enzymes in the bowel lumen, thus preserving the intestinal barrier function.
2.4.12 IGF-1
IGF-1 is known to decrease apoptosis and promote hematopoietic progenitor cell survival. These features, along with its demonstrated safety profile in humans Citation[158], make IGF-1 an attractive candidate for treatment of patients with failing, radiation-induced hematopoietic systems. Mice treated with IGF-1 after lethal TBI show clear and significant hematopoietic recovery acceleration, by protecting HSCs and early marrow progenitors from apoptosis and promoting enhanced proliferation and differentiation within the myeloid compartment: all of which serve to improve overall survival Citation[159].
2.4.13 Anti-ceramide antibody
Ceramide, an inflammatory molecule, is generated on the surface of endothelium, which coalesces to form ceramide-rich platforms that transmit apoptotic signals. Anti-ceramide monoclonal antibody, 2A2, binds to ceramide, preventing platform formation on the surface of endothelial cells, which protects against apoptosis and facilitates recovery of crypt stem cell clonogens in radiation-induced GI syndrome Citation[160]. In brief, 2A2 represents a prototype of a new class of anti-ceramide therapeutics and an effective countermeasure against the radiation GI syndrome.
2.4.14 Histone deacetylase inhibitor, phenylbutyrate
Histone deacetylase inhibitors can suppress cutaneous radiation syndrome and stimulate hematopoiesis. Phenylbutyrate (PB), a novel anti-tumor agent, provided radioprotection against γ-radiation when administered before irradiation and demonstrated a DRF of 1.31 in mouse model. When administered post-irradiation, it provided significant radiomitigation Citation[161]. Prophylactic treatment was associated with significant elevations in neutrophils and platelets. Results demonstrated that PB treatment before radiation can attenuate DNA damage and inhibit radiation-induced apoptosis.
2.4.15 Geldanamycin analog 17-DMAG
Single dose administrations of 17-dimethylamino-ethylamino-17-demethoxygeldanamycin (17-DMAG) increased survival of irradiated mice Citation[162]. Mice pretreated with 17-DMAG showed attenuation of bone marrow aplasia in femurs after irradiation with recovered expressions of CD34 and CD44, and survival in bone marrow cells was observed. 17-DMAG also elevated serum G-CSF levels, decreased serum fms-related tyrosine kinase 3 ligand levels and reduced white blood cell depletion. 17-DMAG ameliorated small intestinal histological damage and promoted recovery of villi and intestinal crypts including stem cells. It was not efficacious if administered after irradiation.
3. Conclusion
Intellectual property rights and exclusive marketing ownership attract the entrepreneurially oriented biomedical research community, thus fostering innovation in the general area of drug development. Major pharmaceutical companies with significant capabilities are generally not interested in developing agents with relatively small market opportunities and limited future revenue potentials. Funding opportunities for the development of agents, like radiation countermeasures, are mostly dependent on government resources. Project BioShield provides new opportunities to improve medical countermeasures against chemical, biological, radiological or nuclear attack through the Radiation and Nuclear Countermeasure Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and BARDA. BARDA provides significant financial support to several start-up companies and other institutions for development of agents demonstrating efficacy in preliminary studies.
Radiation countermeasures are considered ‘emergency need drugs’ and do not have attractive future revenue generation. As a result, these potentially useful medicinals are often granted ‘orphan drug status’ by the US FDA and are defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200,000 people in the US, < 5 per 10,000 people in a community, or that affect > 200,000 persons but are not expected to recover the costs of developing and marketing a drug. Assignment as an ‘orphan drug’ provides the drug’s manufacturer or pharmaceutical company tax reductions and exclusive rights to the cure for a specific indication for a period of 7 years post-approval. It encourages corporations to enter a market where the costs of development are less likely to be recouped, due to the smaller pool of individuals needing the cure.
Usually, the US FDA considers radiation countermeasures under ‘fast track’ approval processes which are designed to facilitate development, expedite the review and approval processes of new treatments of serious or life-threatening conditions. Filling an unmet medical need is defined as providing a therapy where none exists or one that may be superior to currently available therapy. Most countermeasures at more advanced stages of development have received FDA fast-track status.
Although significant advances have been made, investigations of radiation countermeasures have proceeded slowly and with considerable difficulties. Preclinical work is necessary to establish the countermeasure’s potential efficacy and drug mechanism because conventional clinical efficacy trials in humans are not possible due to ethical reasons (except for countermeasures intended for use in cancer patients undergoing radiotherapy). Human efficacy trials are substituted with a very stringent, possibly more difficult, FDA approval pathway: the Animal Efficacy Rule, which requires a good understanding of mechanistic knowledge in two or more animal models predictive of human response Citation[163]. This pathway relies heavily on a large animal model for preclinical safety and efficacy studies, for which resources are limited. Despite massive investment by the US federal government, only five drugs have been approved under Animal Efficacy Rule since 2001 Citation[164].
Demands on large animal research facilities will no doubt increase significantly as a result of the Animal Efficacy Rule. The number of adequate animal models is one main limiting factor for radiation countermeasure development and biomarker discovery; only two large animal models (NHP and canines) have been well characterized Citation[165,166]. NHPs are necessary for preclinical development because of similarities of drug metabolism and physiology between NHPs and humans. Previous experience has shown that canine pharmacokinetics are not always relevant to primate efficacy Citation[167]; consequently, the rhesus macaque is generally the large animal model of choice for toxicity, pharmacokinetics, biomarker, radiation injury and countermeasure investigations. Further, the FDA generally accepts rhesus macaques as the appropriate model for pivotal efficacy testing whenever human efficacy testing cannot be performed and the Animal Efficacy Rule is implemented.
Recently, swine have been suggested as one of the promising species for drug evaluation Citation[165]. The pig model (specifically using the Gottingen minipig) is currently in an initial developmental stage Citation[168]. Additional long-term large animal models for ARS need to be developed and validated to facilitate advanced development of radiation countermeasures.
4. Expert opinion
Since the US FDA has not approved a single drug specifically for ARS arising from radiation exposure, a better public-private partnership is required to boost development. Independent of the FDA’s approval and licensing process, the US Federal government has procured G-CSF (Neupogen), along with GM-CSF (Leukine), to be stockpiled in the Strategic National Stockpile under the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 Citation[169].
During the Joint Meeting of the Medical Imaging Drugs Advisory Committee and the Oncologic Drugs Advisory Committee held on May 3, 2013, committee members overwhelmingly voted (17:1) to support the notion that filgrastim (G-CSF) will produce clinical benefits in humans exposed to radiation doses likely to induce myelosuppression Citation[63]. However, there are reservations; it is likely that most individuals who will survive a nuclear incident would have received radiation doses too low to cause myelosuppression, and filgrastim administration may not provide clinical benefit. The effectiveness of filgrastim after sub-lethal radiation exposure is not well established Citation[170]. In most radiation incidents, there will be partial sparing of bone marrow and stimulation of the victim’s own marrow recovery by G-CSF appears to be a potentially effective strategy.
To refine and optimize filgrastim administration after a nuclear incident scenario, additional information is needed based on the timing of filgrastim administration, use after a variety of radiation doses, and the effects on combined injury (wound, burn, hemorrhage). A recent report suggests that pegylated G-CSF, although effective after TBI, is not able to provide protection in a combined injury (irradiation and burn covering 15% total-body-surface-area) mouse model Citation[77].
To optimize filgrastim administration to radiological/nuclear victims, estimations of radiation dose and the clinical status of the victims would be useful. The stability of filgrastim and the implementation plans in the radiological nuclear incident scenario are concerns as well. Additional apprehension regarding G-CSF’s usefulness as a radiation medical countermeasure also has been raised due to its potential negative effects on radiation-induced lung injury and radiation-induced tumorigenesis Citation[171]. Additionally, critical, pivotal G-CSF studies often have utilized intensive supportive care; in a mass casualty scenario, the analogous treatments will be severely limited Citation[53].
Additional concerns are the side effects of G-CSF administration which include fever, myalgia, respiratory distress, hypoxia, splenomegaly, sickle cell crisis and incidences of Sweet’s syndrome (acute febrile neutropenia dermatosis/skin plaques) Citation[172]. G-CSF treatments have also delayed platelet recovery. Citation[173]. Cancer-prone patients receiving a G-CSF regimen have a slightly increased risk of developing a myeloproliferative-type disorder.
In 2011, a World Health Organization panel of experts convened to develop recommendations for radiation-induced hematopoietic injury management in a mass casualty scenario. The committee strongly indorsed G-CSF or GM-CSF administration within 24 h of exposure (if possible), to victims exposed to doses > 2 Gy, if the neutrophil count is below 0.5 × 109 cells/l and likely to persist for a week or more Citation[174]. It is important to note that study results suggest that benefit was achieved even if G-CSF administration was delayed for several days or even weeks Citation[172]. Pegylated G-CSF could be used as an alternative to G-CSF. CSFs should be administered for 2 – 3 weeks before considering stem cell or progenitor cell transplant therapy.
There are several promising radiation countermeasures under advanced stages of development; the progress of 5-AED, genistein and CBLB502 has slowed due to various reasons. Ex-RAD efficacy studies in the NHP-model are being initiated. Recent developments with HemaMax and OrbeShield indicate that they are ‘on track’ relative to regulatory approval.
Article highlights.
To date, no radiation countermeasure for acute radiation syndrome (ARS) has been approved by the US FDA for human use.
G-CSF may receive US FDA approval. FDA advisory committee members voted that the benefits will outweigh the risks of G-CSF therapy.
Seven promising radiation countermeasures for ARS agents have been granted US FDA investigational new drug status.
Amifostine is the only FDA-approved radioprotective agent for human use; the authorization is for a few defined and limited, clinical indications.
γ-Tocotrienol and AEOL 10150 have demonstrated radioprotective efficacy in nonhuman primates.
Some agents only ameliorate certain manifestations of radiation injury. Investigations using agent combinations with complimentary activities are gaining momentum.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgements
The opinions or assertions contained herein are the professional views of the authors and are not necessarily those of the Department of Defense, USA.
Notes
This box summarizes key points contained in the article
Bibliography
- Carter AB, May MM, Perry WJ. The day after: action following a nuclear blast in a U.S. city. Washington Quarterly 2007;30:19-32
- Benjamin GC, McGeary M, McCutchen SR. Assessing medical preparedness to respond to a terrorist nuclear event: workshop report. The National Academies Press; Washington, DC: 2009
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res 2005;163:115-23
- Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 2004;140:1037-51
- Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 6th edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2006
- Fliedner TM, Dorr DH, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Br J Radiol Suppl 2005;27:1-8
- Hill RP. Radiation effects on the respiratory system. Br J Radiol Suppl 2005;27:75-81
- Moulder JE, Cohen EP. Radiation-induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. Br J Radiol Suppl 2005;27:82-8
- Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res 2004;162:711-28
- Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol 2012;88:296-310
- Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res 2013;54:973-88
- Koenig KL, Goans RE, Hatchett RJ, et al. Medical treatment of radiological casualties: current concepts. Ann Emer Med 2005;45:643-52
- Dumont F, Le Roux A, Bischoff P. Radiation countermeasure agents: an update. Expert Opin Ther Pat 2010;20:73-101
- Seed TM. Radiation protectants: current status and future prospects. Health Phys 2005;89:531-45
- Whitnall MH, Wilhelmsen CL, McKinney L, et al. Radioprotective efficacy and acute toxicity of 5-androstenediol after subcutaneous or oral administration in mice. Immunopharmacol Immunotoxicol 2002;24:595-626
- Stickney DR, Dowding C, Garsd A, et al. 5-androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol 2006;6:1706-13
- Stickney DR, Dowding C, Authier S, et al. 5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol 2007;7:500-5
- Whitnall MH, Villa V, Seed TM, et al. Molecular specificity of 5-androstenediol as a systemic radioprotectant in mice. Immunopharmacol Immunotoxicol 2005;27:1-18
- Whitnall MH, Elliott TB, Harding RA, et al. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int J Immunopharmacol 2000;22:1-14
- Loria RM, Conrad DH, Huff T, et al. Androstenetriol and androstenediol. Protection against lethal radiation and restoration of immunity after radiation injury. Ann N Y Acad Sci 2000;917:860-7
- Whitnall MH, Inal CE, Jackson III WE, et al. In vivo radioprotection by 5-androstenediol: stimulation of the innate immune system. Radiat Res 2001;156:283-93
- Singh VK, Shafran RL, Inal CE, et al. Effects of whole-body gamma irradiation and 5-androstenediol administration on serum G-CSF. Immunopharmacol Immunotoxicol 2005;27:521-34
- Grace MB, Singh VK, Rhee JG, et al. 5-AED enhances survival of irradiated mice in a G-CSF-dependent manner, stimulates innate immune cell function, reduces radiation-induced DNA damage and induces genes that modulate cell cycle progression and apoptosis. J Radiat Res 2012;53:840-53
- Stickney DR, Groothuis JR, Ahlem C, et al. Preliminary clinical findings on NEUMUNE as a potential treatment for acute radiation syndrome. J Radiol Prot 2010;30:687-98
- Soligenix. 2014. Available from: http://www.soligenix.com/prod_def_sgx202.shtml [Last accessed 25 March 2014]
- Georges GE, Kuver RP, Jordan R, et al. Post-exposure oral 17,21-beclomethasone dipropionate (BDP) improves survival in a canine gastrointestinal acute radiation syndrome (GI-ARS) model. 58th Annual Meeting of the Radiation Research Society; San Juan, PR; 2012
- Zenk JL. New therapy for the prevention and prophylactic treatment of acute radiation syndrome. Expert Opin Investig Drugs 2007;16:767-70
- Valachovicova T, Slivova V, Bergman H, et al. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol 2004;25:1389-95
- U.S. Secretary Department of Health and Human Services Uniformed Services University of the Health Sciences. Landauer MR, Seed TM, Srinivasan V, et al. Inventor Isoflavones against radiation-induced mortality. EP1767215; 2007
- Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl toxicol 2003;23:379-85
- Zhou Y, Mi MT. Genistein stimulates hematopoiesis and increases survival in irradiated mice. J Radiat Res 2005;46:425-33
- Landauer M. Radioprotection by the soy isoflavone genistein. In: Arora R, editor. Herbal radiomodulators: applications in medicine, homeland defense and space. CABI Publishing; Wallingford, England: 2008. p. 163-73
- Day RM, Barshishat-Kupper M, Mog SR, et al. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J Radiat Res 2008;49:361-72
- Singh VK, Grace MB, Parekh VI, et al. Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Int Immunopharmacol 2009;9:1401-10
- Davis TA, Clarke TK, Mog SR, Landauer MR. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int J Radiat Biol 2007;83:141-51
- Davis TA, Mungunsukh O, Zins S, et al. Genistein induces radioprotection by hematopoietic stem cell quiescence. Int J Radiat Biol 2008;84:713-26
- Day RM, Davis TA, Barshishat-Kupper M, et al. Enhanced hematopoietic protection from radiation by the combination of genistein and captopril. Int Immunopharmacol 2013;15:348-56
- Humanetics Pharmaceuticals. 2014. Available from: http://www.humaneticscorp.com/ Last accessed 25 January 2014]
- Ha CT, Li XH, Fu D, et al. Genistein nanoparticles protect mouse hematopoietic system and prevent proinflammatory factors after gamma irradiation. Radiat Res 2013;180:316-25
- Cleveland Clinic Foundation Gudkov AV, Didonato JA, Krivokrysenko V. Flagellin related polypeptide and uses thereof. WO/2006/069198; 2007
- Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320:226-30
- Vijay-Kumar M, Aitken JD, Sanders CJ, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol 2008;180:8280-5
- Krivokrysenko V, Toshov I, Gleiberman AS, et al. Single injection of novel medical radiation countermeasure CBLB502 rescues nonhuman primates within broad time window after lethal irradiation. 56th Annual Meeting of the Radiation Research Society. Maui, Hawaii; 2010
- Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther 2012;343:497-508
- Cleveland BioLabs, Inc. 2014. Available from: http://www.cbiolabs.com/ [Last accessed 2014]
- Neumedicine. 2014. Available from: http:/www.neumedicines.com/about.shtml [Last accessed 27 March 2014]
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002;13:155-68
- Chen T, Burke KA, Zhan Y, et al. IL-12 facilitates both the recovery of endogenous hematopoiesis and the engraftment of stem cells after ionizing radiation. Exp Hematol 2007;35:203-13
- Basile LA, Gallaher TK, Shibata D, et al. Multilineage hematopoietic recovery with concomitant antitumor effects using low dose Interleukin-12 in myelosuppressed tumor-bearing mice. J Transl Med 2008;6:26
- Basile LA, Ellefson D, Gluzman-Poltorak Z, et al. HemaMax, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS One 2012;7:e30434
- Xiong GL, Zhao Y, Xing S, et al. Radiation protection effect of rhIL-12 on monkey hematopoietic system. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013;21:150-4
- Ellefson D, Galalaher T, Gluzman-Poltorak Z, et al. Mitigation of radiation combined injury (RCI) by interleukin-12. 14th International Congress of Radiation Research; Warsaw, Poland; 2011
- Farese AM, Cohen MV, Katz BP, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res 2013;179:89-100
- Gluzman-Poltorak Z, Mendonca SR, Vainstein V, et al. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol 2014;7:31
- Ghosh SP, Perkins MW, Hieber K, et al. Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms. Radiat Res 2009;171:173-9
- Suman S, Maniar M, Fornace AJ Jr, Datta K. Administration of ON 01210.Na after exposure to ionizing radiation protects bone marrow cells by attenuating DNA damage response. Radiat Oncol 2012;7:6
- Suman S, Datta K, Doiron K, et al. Radioprotective effects of ON 01210.Na upon oral administration. J Radiat Res 2012;53:368-76
- Kumar R. Radioprotection and radiomitigation properties of Ex-Rad upon oral administration. 56th Annual Meeting of the Radiation Research Society; Maui, Hawaii; 2010
- Ghosh SP, Kulkarni S, Perkins MW, et al. Amelioration of radiation-induced hematopoietic and gastrointestinal damage by Ex-RAD(R) in mice. J Radiat Res 2012;53:526-36
- Kang AD, Cosenza SC, Bonagura M, et al. ON01210.Na (Ex-RAD(R)) mitigates radiation damage through activation of the AKT pathway. PLoS One 2013;8:e58355
- Onconova Therapeutics, Inc. 2014. Available from: http://www.onconova.com/product-pipeline/recilisib.php [Last accessed 28 March 2014]
- HHS boosts stockpile of products to treat acute radiation syndrome. 2013. Available from: http://www.hhs.gov/news/press/2013pres/09/20130926a.html [Last accessed 2014]
- U.S. Food and Drug Administration. FDA Advisory Committee Briefing Document: a Joint Meeting of the Medical Imaging Drugs Advisory Committee and the Oncologic Drugs Advisory Committee. 2013. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM350151.pdf [Last accessed 5 February 2014]
- Hosoi Y, Kurishita A, Ono T, Sakamoto K. Effect of recombinant human granulocyte colony-stimulating factor on survival in lethally irradiated mice. Acta Oncol 1992;31:59-63
- Sureda A, Kadar E, Valls A, Garcia-Lopez J. Granulocyte colony-stimulating factor administered as a single intraperitoneal injection modifies the lethal dose95/30 in irradiated B6D2F1 mice. Haematologica 1998;83:863-4
- Patchen ML, MacVittie TJ. Granulocyte colony-stimulating factor and amifostine (Ethyol) synergize to enhance hemopoietic reconstitution and increase survival in irradiated animals. Semin Oncol 1994;21:26-32
- Tanikawa S, Nose M, Aoki Y, et al. Effects of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood 1990;76:445-9
- Mac Vittie TJ, Monroy RL, Farese AM, et al. Cytokine therapy in canine and primate models of radiation-induced marrow aplasia. Behring Inst Mitt 1991;90:1-13
- Nash RA, Schuening FG, Seidel K, et al. Effect of recombinant canine granulocyte-macrophage colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood 1994;83:1963-70
- MacVittie TJ, Monroy RL, Patchen ML, Souza LM. Therapeutic use of recombinant human G-CSF (rhG-CSF) in a canine model of sublethal and lethal whole-body irradiation. Int J Radiat Biol 1990;57:723-36
- Schuening FG, Appelbaum FR, Deeg HJ, et al. Effects of recombinant canine stem cell factor, a c-kit ligand, and recombinant granulocyte colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood 1993;81:20-6
- Schuening FG, Storb R, Goehle S, et al. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood 1989;74:1308-13
- Moroni M, Ngudiankama BF, Christensen C, et al. The Gottingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body gamma-irradiation. Int J Radiat Oncol Biol Phys 2013;86:986-92
- Cary LH, Ngudiankama BF, Salber RE, et al. Efficacy of radiation countermeasures depends on radiation quality. Radiat Res 2012;177:663-75
- Chua HL, Plett PA, Sampson CH, et al. Survival efficacy of the PEGylated G-CSFs Maxy-G34 and neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Phys 2014;106:21-38
- Farese AM, Cohen MV, Stead RB, et al. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res 2012;178:403-13
- Kiang JG, Zhai M, Liao PJ, et al. Pegylated G-CSF inhibits blood cell depletion, increases platelets, blocks splenomegaly, and improves survival after whole-body ionizing irradiation but not after irradiation combined with burn. Oxid Med Cell Longev 2014;2014:481392
- Atkinson K, Matias C, Guiffre A, et al. In vivo administration of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF, interleukin-1 (IL-1), and IL-4, alone and in combination, after allogeneic murine hematopoietic stem cell transplantation. Blood 1991;77:1376-82
- Monroy RL, Skelly RR, Taylor P, et al. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol 1988;16:344-8
- Tanikawa S, Nakao I, Tsuneoka K, Nara N. Effects of recombinant granulocyte colony-stimulating factor (rG-CSF) and recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) on acute radiation hematopoietic injury in mice. Exp Hematol 1989;17:883-8
- Waddick KG, Song CW, Souza L, Uckun FM. Comparative analysis of the in vivo radioprotective effects of recombinant granulocyte colony-stimulating factor (G-CSF), recombinant granulocyte-macrophage CSF, and their combination. Blood 1991;77:2364-71
- Patchen ML, Fischer R, MacVittie TJ, et al. Mast cell growth factor (C-kit ligand) in combination with granulocyte-macrophage colony-stimulating factor and interleukin-3: in vivo hemopoietic effects in irradiated mice compared to in vitro effects. Biotherapy 1993;7:13-26
- Nothdurft W, Selig C, Fliedner TM, et al. Haematological effects of rhGM-CSF in dogs exposed to total-body irradiation with a dose of 2.4 Gy. Int J Radiat Biol 1992;61:519-31
- Selig C, Nothdurft W, Kreja L, Fliedner TM. Influence of combined treatment with interleukin 1 and erythropoietin or GM-CSF and erythropoietin on the regeneration of hemopoiesis in the dog after total body irradiation – a preliminary report. Behring Inst Mitt 1991(90):86-92
- Farese AM, Williams DE, Seiler FR, MacVittie TJ. Combination protocols of cytokine therapy with interleukin-3 and granulocyte-macrophage colony-stimulating factor in a primate model of radiation-induced marrow aplasia. Blood 1993;82:3012-18
- Neelis KJ, Hartong SC, Egeland T, et al. The efficacy of single-dose administration of thrombopoietin with coadministration of either granulocyte/macrophage or granulocyte colony-stimulating factor in myelosuppressed rhesus monkeys. Blood 1997;90:2565-73
- Rasey JS, Spence AM, Badger CC, et al. Specific protection of different normal tissues. Pharmacol Ther 1988;39:33-43
- Glover D, Fox KR, Weiler C, et al. Clinical trials of WR-2721 prior to alkylating agent chemotherapy and radiotherapy. Pharmacol Ther 1988;39:3-7
- Weiss JF. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect 1997;105(Suppl 6):1473-8
- MedImmune. 2013. Available from: http://www.medimmune.com/docs/default-source/pdfs/prescribing-information-for-amifostine.pdf [Last accessed 30 September 2013]
- Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs 2001;61:641-84
- Seed TM, Inal CE, Singh VK. Radioprotection of hematopoietic progenitors by low dose amifostine prophylaxis. Int J Radiat Biol 2014;90:594-604
- Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med 2007;28:692-728
- Baliarsingh S, Beg ZH, Ahmad J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005;182:367-74
- Qureshi AA, Burger WC, Peterson DM, Elson CE. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem 1986;261:10544-50
- Berbee M, Fu Q, Boerma M, et al. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog gamma-tocotrienol: evidence of a role for tetrahydrobiopterin. Int J Radiat Oncol Biol Phys 2011;79:884-91
- Berbee M, Fu Q, Boerma M, et al. Gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res 2009;171:596-605
- Ghosh SP, Kulkarni S, Hieber K, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol 2009;85:598-606
- Kulkarni S, Ghosh SP, Satyamitra M, et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res 2010;173:738-47
- Kulkarni SS, Cary LH, Gambles K, et al. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int Immunopharmacol 2012;14:495-503
- Suman S, Datta K, Chakraborty K, et al. Gamma tocotrienol, a potent radioprotector, preferentially upregulates expression of anti-apoptotic genes to promote intestinal cell survival. Food Chem Toxicol 2013;60:488-96
- Kulkarni S, Singh PK, Ghosh SP, et al. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine 2013;62:278-85
- Li XH, Fu D, Latif NH, et al. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010;95:1996-2004
- Li XH, Ghosh SP, Ha CT, et al. Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat Res 2013;180(6):649-57
- Satyamitra M, Ney P, Graves J III, et al. Mechanism of radioprotection by delta-tocotrienol: pharmacokinetics, pharmacodynamics and modulation of signalling pathways. Br J Radiol 2012;85:e1093-103
- Satyamitra MM, Kulkarni S, Ghosh SP, et al. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin E isoform delta-tocotrienol. Radiat Res 2011;175:736-45
- Singh VK, Wise SY, Scott JR, et al. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci 2014;98:113-22
- Gridley DS, Makinde AY, Luo X, et al. Radiation and a metalloporphyrin radioprotectant in a mouse prostate tumor model. Anticancer Res 2007;27:3101-9
- Pearlstein RD, Higuchi Y, Moldovan M, et al. Metalloporphyrin antioxidants ameliorate normal tissue radiation damage in rat brain. Int J Radiat Biol 2010;86:145-63
- Zhang Y, Zhang X, Rabbani ZN, et al. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys 2012;83:740-8
- Aeolus Pharmaceuticals. 2013. Available from: http://www.aeoluspharma.com/ [Last accessed 30 September 2013]
- Batinic-Haberle I, Tovmasyan A, Roberts E, et al. SOD therapeutics: Latest insights into their structure-activity relationships and impact upon the cellular redox-based pathways. Antioxid Redox Signal 2014;20(15):2372-415
- Orrell RW. AEOL-10150 (Aeolus). Curr Opin Investig Drugs 2006;7:70-80
- Garofalo MC, Ward AA, Farese AM, et al. A Pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys 2014;106:73-83
- Singh VK, Christensen J, Fatanmi OO, et al. Myeloid Progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res 2012;177:781-91
- Singh VK, Elliott TB, Mandalam R, et al. Myeloid progenitors mitigate radiation injury and improve intestinal integrity after whole-body irradiation. NATO RTO HFM-223 Symposium “Biological effects of ionizing radiation exposure and countermeasures: current status and future perspectives”; 8-10 October 2012; Ljubljana, Slovenia; p. 1-13
- Cellerant therapeutics. 2013. Available from: http://www.cellerant.com/ [Last accessed 26 September 2013]
- Satyamitra M, Lombardini E, Graves J III, et al. A TPO receptor agonist, ALXN4100TPO, mitigates radiation-induced lethality and stimulates hematopoiesis in CD2F1 mice. Radiat Res 2011;175:746-58
- Satyamitra M, Lombardini E, Peng T, et al. Preliminary nonclinical toxicity, pharmacokinetics, and pharmacodynamics of ALXN4100TPO, a thrombopoietin receptor agonist, in CD2F1 mice. Int J Toxicol 2013;32:100-12
- Cai Y, Wang W, Liang H, et al. Keratinocyte growth factor pretreatment prevents radiation-induced intestinal damage in a mouse model. Scand J Gastroenterol 2013;48:419-26
- Finch PW, Mark Cross LJ, McAuley DF, Farrell CL. Palifermin for the protection and regeneration of epithelial tissues following injury: new findings in basic research and pre-clinical models. J Cell Mol Med 2013;17:1065-87
- Vadhan-Raj S, Goldberg JD, Perales MA, et al. Clinical applications of palifermin: amelioration of oral mucositis and other potential indications. J Cell Mol Med 2013;17:1371-84
- Epperly MW, Bray JA, Krager S, et al. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys 1999;43:169-81
- Epperly MW, Sikora CA, DeFilippi SJ, et al. Manganese superoxide dismutase (SOD2) inhibits radiation-induced apoptosis by stabilization of the mitochondrial membrane. Radiat Res 2002;157:568-77
- Guo H, Seixas-Silva JA Jr, Epperly MW, et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res 2003;159:361-70
- Epperly MW, Wang H, Jones JA, et al. Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res 2011;175:759-65
- Robbins ME, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys 2006;64:6-12
- Robbins ME, Hopewell JW. Physiological factors effecting renal radiation tolerance: a guide to the treatment of late effects. Br J Cancer Suppl 1986;7:265-7
- Davis TA, Landauer MR, Mog SR, et al. Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation. Exp Hematol 2010;38:270-81
- Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res 2011;175:29-36
- Kma L, Gao F, Fish BL, et al. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res 2012;53:10-17
- Medhora M, Gao F, Jacobs ER, Moulder JE. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology 2012;17:66-71
- Ghosh SN, Zhang R, Fish BL, et al. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys 2009;75:1528-36
- Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol 2007;17:141-8
- Ward WF, Kim YT, Molteni A, Solliday NH. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys 1988;15:135-40
- Ward WF, Molteni A, Ts’ao C, Hinz JM. The effect of Captopril on benign and malignant reactions in irradiated rat skin. Br J Radiol 1990;63:349-54
- Cohen EP, Fish BL, Moulder JE. Treatment of radiation nephropathy with captopril. Radiat Res 1992;132:346-50
- Moulder JE, Cohen EP, Fish BL, Hill P. Prophylaxis of bone marrow transplant nephropathy with captopril, an inhibitor of angiotensin-converting enzyme. Radiat Res 1993;136:404-7
- Cohen EP, Irving AA, Drobyski WR, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys 2008;70:1546-51
- Charrier S, Michaud A, Badaoui S, et al. Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 2004;104:978-85
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005;4:1201-15
- Reed GA, Arneson DW, Putnam WC, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3’-diindolylmethane. Cancer Epidemiol Biomarkers Prev 2006;15:2477-81
- Reed GA, Sunega JM, Sullivan DK, et al. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3’-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev 2008;17:2619-24
- Heath EI, Heilbrun LK, Li J, et al. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3’- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res 2010;2:402-11
- Del Priore G, Gudipudi DK, Montemarano N, et al. Oral diindolylmethane (DIM): pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol Oncol 2010;116:464-7
- Fan S, Meng Q, Xu J, et al. DIM (3,3’-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc Natl Acad Sci USA 2013;110:18650-5
- Kumar A, Kochar A, Sharma D, et al. Antimutagenic and radioprotective effects of oltipraz. 14th International Congress of Radiation Research; Warsaw, Poland; 2011
- Kim SG, Nam SY, Kim CW. In vivo radioprotective effects of oltipraz in gamma-irradiated mice. Biochem Pharmacol 1998;55:1585-90
- Lazo JS, Sharlow ER, Epperly MW, et al. Pharmacologic profiling of phosphoinositide 3-kinase inhibitors as mitigators of ionizing radiation-induced cell death. J Pharmacol Exp Ther 2013;347:669-80
- Maclachlan T, Narayanan B, Gerlach VL, et al. Human fibroblast growth factor 20 (FGF-20; CG53135-05): a novel cytoprotectant with radioprotective potential. Int J Radiat Biol 2005;81:567-79
- Okunieff P, Wu T, Huang K, Ding I. Differential radioprotection of three mouse strains by basic or acidic fibroblast growth factor. Br J Cancer Suppl 1996;27:S105-8
- Casey-Sawicki K, Zhang M, Kim S, et al. A basic fibroblast growth factor analog for protection and mitigation against acute radiation syndromes. Health Phys 2014;106:704-12
- Ma J, Hou Y, Han D, et al. Fibroblast growth factor-peptide promotes bone marrow recovery after irradiation. Adv Exp Med Biol 2013;765:155-61
- Bhanja P, Saha S, Kabarriti R, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One 2009;4:e8014
- Kiss GN, Lee SC, Fells JI, et al. Mitigation of radiation injury by selective stimulation of the LPA(2) receptor. Biochim Biophys Acta 2013;1831:117-25
- Fu Q, Berbee M, Boerma M, et al. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res 2009;171:698-707
- Fu Q, Berbee M, Wang W, et al. Preclinical evaluation of Som230 as a radiation mitigator in a mouse model: postexposure time window and mechanisms of action. Radiat Res 2011;175:728-35
- Rosenbloom AL. Mecasermin (recombinant human insulin-like growth factor I). Adv Ther 2009;26:40-54
- Zhou D, Deoliveira D, Kang Y, et al. Insulin-like growth factor 1 mitigates hematopoietic toxicity after lethal total body irradiation. Int J Radiat Oncol Biol Phys 2013;85:1141-8
- Rotolo J, Stancevic B, Zhang J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest 2012;122:1786-90
- Miller AC, Cohen S, Stewart M, et al. Radioprotection by the histone deacetylase inhibitor phenylbutyrate. Radiat Environ Biophys 2011;50:585-96
- Lu X, Nurmemet D, Bolduc DL, et al. Radioprotective effects of oral 17-dimethylaminoethylamino-17-demethoxygeldanamycin in mice: bone marrow and small intestine. Cell Biosci 2013;3:36
- Gronvall GK, Trent D, Borio L, et al. The FDA animal efficacy rule and biodefense. Nat Biotechnol 2007;25:1084-7
- Guidance for industry: product developoment under the animal rule. 2014. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf [Last accessed 2014]
- Augustine AD, Gondre-Lewis T, McBride W, et al. Animal models for radiation injury, protection and therapy. Radiat Res 2005;164:100-9
- Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res 2010;173:557-78
- Ahlem CN, White SK, Page TM, Frincke JM. Differential metabolism of androst-5-ene-3beta,17beta-diol between rats, canines, monkeys and humans. Steroids 2011;76:669-74
- Shim S, Jang WS, Lee SJ, et al. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res 2014;181:387-95
- U.S. Food and Drug Administration. About the pandemic and all-hazards preparedness reauthorization Act of 2013 (PAHPRA), emergency preparedness and response. 2014. Available from: http://www.fda.gov/emergencypreparedness/medicalcountermeasures/ucm346195.htm [Last accessed 15 February 2014]
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine 2015;71:22-37
- Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets 2013;14:1347-56
- Reeves G. Overview of use of G-CSF and GM-CSF in the treatment of acute radiation injury. Health Phys 2014;106:699-703
- International Atomic Energy Agency. The radiological accident in Istanbul. 2000. Available from: http://www-pub.iaea.org/books/IAEABooks/6071/The-Radiological-Accident-in-Istanbul [Last accessed 2014]
- Dainiak N, Gent RN, Carr Z, et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep 2011;5:202-12

