Keywords::
1. Introduction
In this issue, the authors provided a comprehensive review of mTORC1 inhibitors (sirolimus (rapamycin), temsirolimus, everolimus and ridaforolimus) and dual kinase inhibitors (OSI-027, AZD8055, AZD2014 and INK128). The mammalian target of rapamycin (mTOR) pathway is a crucial target in new drug development. Since first being described in the 1990s, the mTOR pathway has been greatly researched Citation[1]. In many different cancers, activations of PI3K/AKT/mTOR signalling through mutations of pathway components play a crucial role in deregulation of proliferation, in angiogenesis and in resistance to apoptosis resulting in cell survival and resistance to chemotherapy and radiotherapy Citation[2-6].
Activation of the mTOR complex, which is mainly located in the nuclear fraction of both normal and neoplastic cells, prompts resting cells to increase translation of a subset of mRNA Citation[7]. These translated proteins escalate cell cycle progression from G1 to S phase. In response to mitogenic signals, the mTOR complex is phosphorylated, which leads to mRNA translation in the presence of favourable nutrient environments Citation[8,9]. In vitro, mTOR-inhibitor-treated cells expressed mTOR inactivation, downregulation of translation, G1 arrest, accumulation of glycogen stores and altered transcription patterns. Strikingly, these conditions were similar to those observed under starvation Citation[9]. More recent studies have demonstrated that mTOR is involved in regulating many aspects of cell growth, including organisation of the actin cytoskeleton, membrane traffic, protein degradation, protein kinase C signalling, ribosome biogenesis and transcription Citation[10].
2. FDA-approved mTOR inhibitors
Currently, there are four mTORC1 inhibitors: sirolimus, temsirolimus, everolimus and ridaforolimus Citation[11-13]. Sirolimus is approved as an immunosuppressive agent for patients with renal transplants Citation[14]. Temsirolimus is an ester of the macrocyclic immunosuppressive agent sirolimus. Temsirolimus has been shown to inhibit the growth of a wide range of histologically diverse tumours, including adrenocortical carcinoma Citation[15], the Ewing's sarcoma family of tumours Citation[16], central nervous system cancers Citation[17], mantle cell lymphoma Citation[18], breast cancer Citation[19] and renal cell carcinoma Citation[20]. Temsirolimus is Food and Drug Administration (FDA)-approved for renal cell carcinoma Citation[12,20].
Everolimus in combination with exemestane has been FDA-approved for hormone receptor-positive metastatic breast cancer based on the BOLERO-2 trial Citation[13,21]. Everolimus is also FDA-approved to treat patients with advanced renal cell carcinoma and neuroendocrine tumours Citation[22-25]. Ridaforolimus has not yet been approved by the FDA, however its activity has been observed in sarcoma patients Citation[26,27].
3. Toxicity associated with mTOR inhibitors
Some of the common toxicities associated with mTOR inhibitors include clinical toxicities such as asthenia, rash and mucositis and laboratory abnormalities such as anaemia, hyperglycaemia and hyperlipidaemia Citation[12,15,16,28-30]. Pneumonitis, even though it is rare, is a clinically noteworthy complication of treatment with mTOR inhibitors Citation[31]. In general, single-agent mTOR inhibitors are well tolerated; however, mTOR in combination with other agents may cause significant side effects depending on the mechanisms of the second drug. Mucositis and endocrine complications have been observed in patients who received temsirolimus and insulin-like-growth factor receptor (IGF-1R) inhibitor Citation[15,16,29]. Even though mTOR combination therapy causes hyperlipidaemia and hyperglycaemia, patients with diabetes and high cholesterol should not be excluded from combination-therapy clinical trials, as long as their blood sugar and lipids are well controlled. In clinical trials combining MEK inhibitors and mTOR inhibitors, the most frequent toxicities were mucositis, thrombocytopenia and skin rash Citation[32,33]. It is highly recommended to avoid strong CYP3A4 modifiers when using mTOR inhibitors since CYP3A4 is the primary enzyme responsible for metabolism of this class of drugs Citation[34].
4. Resistance to mTOR inhibition and strategies for overcoming resistance
Understanding the mechanism of resistance to mTOR inhibition is critical. Various pathways have been delineated as responsible for resistance to mTOR inhibition. There are multiple strategies for overcoming this resistance.
4.1 Dual inhibition of mTOR complexes
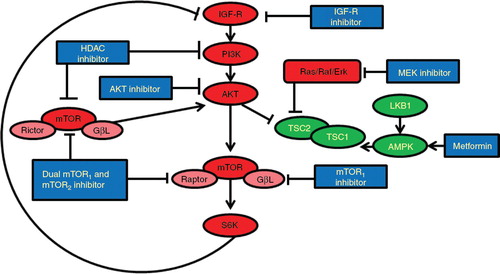
Since mTOR exists in two complexes – mTOR complex 1 (mTORC1) containing Raptor, which is rapamycin sensitive, and mTOR complex 2 (mTORC2) containing Rictor, which is rapamycin insensitive – there is an opportunity for dual inhibition Citation[35]. mTORC1 phosphorylates 4-EPB1 and p70S6 kinase, which results in cell cycle progression. mTORC2 has been shown to directly phosphorylate and activate the upstream kinase AKT Citation[35,36]. Inhibition of mTORC1 impedes the negative feedback loop between S6 kinase and insulin receptor substrate 1 (IRS-1), resulting in increased PI3K (phosphatidylinositol 3-kinase) and AKT activity, thereby limiting the activity of mTORC1 inhibitors Citation[35]. A dual kinase inhibits both mTORC1 and mTORC2 complexes (). By controlling both S6K and AKT, dual kinase inhibitors provide a broader spectrum of activity than does mTORC1 inhibitor alone Citation[35,37,38].
4.2 Inhibition of the PI3K/AKT/mTOR and MAPK pathways
The PI3K pathway and the mitogen-activated protein kinase (MAPK) pathway share common upstream activators. Both activated by oncogenic RAS, these two pathways are significantly interconnected by feedback loops, and one pathway provides compensatory signalling when the other is inhibited Citation[39,40]. The inactivation of either the MAPK or PI3K pathway can lead to incomplete tumour growth inhibition. Parallel inhibition of the PI3K/AKT/mTOR and MAPK pathways leads to synergistic increases in apoptosis in both phosphatase and tensin homolog (PTEN)-mutant and wild-type cells Citation[41,42]. In vivo studies have shown marked synergies when PI3K/AKT/mTOR inhibitors are combined with MEK inhibitors in KRAS-mutant cancers Citation[43]. Thus, combining an mTOR inhibitor and a MEK inhibitor is an appropriate strategy to enhance mTOR-targeted anti-cancer therapy ().
4.3 Inhibition of the PI3K/AKT/mTOR and IGF pathways
AKT activation plays a crucial role in many cellular processes, including cancer cell survival, proliferation and growth Citation[2]. Thus, an unfavourable consequence of mTOR inhibition is the induction of phosphorylated AKT, in turn causing resistance to mTOR inhibition Citation[2]. Several studies have shown that mTOR-induced AKT activation is due to the release of feedback inhibition mediated by the IGF pathway. IGF-1R inhibitors can reduce AKT phosphorylation induced by mTOR inhibitors. AKT activation is related to the escape/resistance mechanism of mTOR inhibitors, but combination studies with mTOR inhibitors and IGF-1R inhibitors suggest additive anti-tumour effects compared with treatment with single agents alone (). Furthermore, as mTOR is involved in signal transduction downstream of the IGF pathway, the combination potentially enhances the activity of IGF-1R inhibitors Citation[29]. Therefore, simultaneous inhibition of both pathways could lead to a more effective anti-tumour strategy when compared with individual pathway blockade Citation[15,16].
Moreover, AKT activation is pivotal in resistance to mTOR inhibition, there are several AKT inhibitors (MK-2206, RX-0201, PBI-05204, GSK2141795) currently being investigated in clinical trials as single agent or in combination with other mTOR inhibitors or chemotherapy Citation[44].
4.4 Metformin in combination with mTOR inhibitors
The combination of rapamycin and metformin, an anti-diabetic drug, was shown to expand the therapeutic window of normal human cell types (RPE, NKE, WI-38t cells) exposed to paclitaxel and nocodazole, both of which are microtubule inhibitors Citation[45]. The drug combination accomplished these results by selectively inducing G1 and G2 arrest in normal cells. Metformin and rapamycin protected normal cells in low-glucose conditions, but the combination was cytotoxic to cancer cells Citation[45].
This combination is attractive because the mTOR inhibitor and metformin appear to inhibit the mTOR pathway by different mechanisms Citation[46]. mTORC1 inhibitors inhibit mTOR activity by binding to FKBP12, which can result in the unintended upregulation of AKT through a positive feedback loop that might undesirably accelerate cell proliferation. Metformin inhibits the mTOR pathway through upstream activation of 5′-adenosine monophosphate-activated protein kinase (AMPK) Citation[46]. In contrast to mTOR inhibitors, metformin decreases AKT activation. Via AMPK activation, metformin phosphorylates an inhibitory site of IRS-1, which is associated with decreased AKT activation, reduced mTOR activation and reduced feedback inhibition Citation[46].
Metformin, independent of AMPK, also induces mTOR inhibition and cell cycle arrest through REDD1 (a negative regulator of mTOR) in LNCaP (androgen-sensitive human prostate adenocarcinoma) cell lines Citation[47]. In fact, metformin upregulates REDD1, leading to an anti-proliferative effect. Metformin-induced cell cycle arrest and cyclin D1 decrease are not dependent on AMPK in LNCaP cell lines Citation[47]. Therefore, in addition to improving resistance to mTOR inhibition, metformin augments further independent inhibition of the mTOR pathway Citation[47].
In addition, AMPK activation counteracts the inhibitory effect of the Ras/Raf/ERK pathway on tuberous sclerosis complex 2 (TSC2) Citation[48]. These results support the rationale that dual inhibition of the AMPK/TSC2/mTOR pathway through combining metformin and an mTOR inhibitor will enhance the anti-tumour activity Citation[48]. Furthermore, metformin improved hyperglycaemia caused by mTOR inhibitor Citation[29]. Both metformin and the mTOR inhibitor significantly influence intracellular energy homeostasis and interfere with autophagy mechanisms Citation[49]. Thus, combining an mTOR inhibitor and metformin may be an appropriate strategy to enhance mTOR-targeted anti-cancer therapy ().
4.5 Combination of histone deacetylase inhibitors and mTOR inhibitors
Histone deacetylases (HDACs) are enzymes that regulate chromatin structure and function through the catalysis of the removal of acetyl modifications from the lysine residues of histones Citation[50]. Inhibition of HDACs reverses aberrant epigenetic changes associated with malignancy Citation[51], and HDAC inhibitors possess anti-tumour activities against multiple cancers Citation[52]. The HDAC inhibitor SAHA (suberoylanilide hydroxamic acid) directly diminished the kinase activity of PI3K in several cell lines, including PC3 (prostate cancer) and Jeko1 (mantle cell lymphoma) in vitro Citation[53]. Inhibiting PI3K activity in this manner could counteract the paradoxical increase in phosphorylated-AKT in response to mTOR inhibition. Inhibiting HDACs with LBH589 diminished resistance to mTOR inhibition by decreasing AKT activation by two additional mechanisms: activating protein phosphatase PP1 and inhibiting the mTORC2 complex Citation[54]. A combination of a novel HDAC inhibitor (FK228) and a PI3K/AKT pathway inhibitor (LY294002) showed synergy in cytotoxic activity in lung adenocarcinoma cell lines Citation[55]. Adding an HDAC inhibitor to an mTOR inhibitor substantially suppressed survivin levels and generated better tumour growth inhibition than did the single-agent mTOR inhibitor () Citation[56].
5. mTOR inhibition as a tool to overcome resistance to other drugs
Beyond their function of inhibiting mTOR complexes, mTOR inhibitors play an important role in overcoming resistance to other drugs, such as anti-VEGF therapies and anti-EGFR treatment.
5.1 Resistance to anti-angiogenesis treatment
mTOR inhibitors can improve the anti-angiogenic activity of anti-VEGF therapies by several mechanisms. First, mTOR inhibitors inhibit endothelial cell function in neoangiogenesis. Additionally, they inhibit VEGF production induced by hypoxia-stimulated hypoxia-inducible factor 1-α (HIF-1α) Citation[57]. Increased HIF-1α is associated with increased expression of VEGF, aggressive tumour growth and poor prognosis. This phenomenon is commonly observed as a resistance mechanism in tumours treated with anti-VEGF therapy. HIF-1α inhibition in combination with anti-angiogenic therapy is a promising strategy for targeting tumour resistance Citation[58]. This important property of mTOR inhibitors makes them ideal candidates for combination therapy with anti-angiogenic agents.
5.2 Resistance to anti-EGFR treatment
The combination of an EGFR inhibitor and an mTOR inhibitor has the reciprocal benefit of improving resistance to each other. Cancer cell lines exposed to mTOR inhibition have demonstrated increased EGFR/Ras/Raf/Erk/MEK pathway signalling Citation[59]. EGFR possesses kinase-independent activity that induces cancer cell survival by preventing autophagic cell death and maintaining intracellular glucose levels through interaction and stabilisation of the sodium/glucose co-transporter 1 (SGLT1) Citation[60]. Downregulation of EGFR led to a loss of SGLT1 expression, resulting in lower intracellular glucose concentration Citation[61]. Therefore, adding an EGFR inhibitor to an mTOR inhibitor will provide a strategy to overcome resistance.
mTOR is a central player in the autophagy pathway, and downregulation of the PI3K pathway induces autophagy Citation[62]. In glioma cell lines resistant to EGFR tyrosine kinase inhibitors, increased activities of the IGF pathway and of its downstream effectors, PI3K/AKT/mTOR, were observed Citation[63]. Simultaneous inhibition of the EGFR and PI3K/AKT/mTOR pathways inhibited tumour cells that were resistant to small-molecule inhibitors of EGFR Citation[63-65]. Therefore, adding mTOR inhibition to an anti-EGFR antibody/small molecule will provide a strategy for overcoming resistance.
6. Conclusion
In conclusion, there are several ways to improve efficacy of mTOR inhibition. Combination of mTOR inhibitors with other agents that decrease resistance to mTOR inhibition can improve the efficacy of mTOR inhibition. Additionally, mTOR inhibition can improve the therapeutic effect of anti-VEGF therapies and anti-EGFR therapies. With combination therapies, toxicities may be a major obstacle. Hence, it is of great importance to devise an optimal dosing schedule, maintain appropriate sequence of drug administration and optimize management of side effects. Further understanding of resistance and response mechanisms of mTOR inhibitors is critical. Biopsies at baseline before initiation of the treatment and at the time of progression are necessary and may present biological evidence of mutational changes in tumour specimens. Gene sequencing platforms may provide insight into resistance mechanisms, thereby providing more opportunities to improve the efficacy of this class of drugs.
Declaration of interest
The author states no conflict of interest and has received no payment in preparation of this manuscript.
Bibliography
- Harris TE, Lawrence Jr JC. TOR signaling. Sci Signal 2003;2003:re15
- O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006;66:1500-8
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2010;141:1117-34
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489-501
- Vignot S, Faivre S, Aguirre D, et al. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 2005;16:525-37
- Strimpakos AS, Karapanagiotou EM, Saif MW, et al. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev 2009;35:148-59
- Zhang X, Shu L, Hosoi H, et al. Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J Biol Chem 2002;277:28127-34
- Shi Y, Yan H, Frost P, et al. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 2005;4:1533-40
- Barbet NC, Schneider U, Helliwell SB, et al. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell 1996;7:25-42
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 2000;103:253-62
- MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001;71:271-80
- Kwitkowski VE, Prowell TM, Ibrahim A, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist 2010;15:428-35
- Beaver JA, Park BH. The BOLERO-2 trial: the addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol 2012;8:651-7
- Pirsch JD, Miller J, Deierhoi MH, et al. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 1997;63:977-83
- Naing A, LoRusso P, Fu S, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer 2013;108:826-30
- Naing A, LoRusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res 2012;18:2625-31
- Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 2005;23:5294-304
- Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 2005;23:5347-56
- Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 2005;23:5314-22
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56
- Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 2008;26:4311-18
- Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol 2010;28:69-76
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23
- Mita M, Sankhala K, Abdel-Karim I, et al. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs 2008;17:1947-54
- Mita MM, Poplin E, Britten CD, et al. Phase I/IIa trial of the mammalian target of rapamycin inhibitor ridaforolimus (AP23573; MK-8669) administered orally in patients with refractory or advanced malignancies and sarcoma. Ann Oncol 2013;24:1104-11
- Busaidy NL, Farooki A, Dowlati A, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol 2012;30:2919-28
- Naing A, Kurzrock R, Burger A, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res 2011;17:6052-60
- Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res 2006;12:5755-63
- Iacovelli R, Palazzo A, Mezi S, et al. Incidence and risk of pulmonary toxicity in patients treated with mTOR inhibitors for malignancy. A meta-analysis of published trials. Acta Oncol (Madr) 2012;51:873-9
- Naing A, Mita M, Komarnitsky P, et al. 608 Phase i dose-escalation trial of a selective oral MEK1/2 inhibitor, PIMASERTIB (MSC1936369B), combined with an mTOR inhibitor, temsirolimus, in patients with advanced solid tumors. Eur J Cancer 2012;48(Suppl 6):187
- Jeffrey R, Infante AP, Suzanne F, et al. A phase IB study of the MEK inhibitor GSK1120212 combined with everolimus in patients with solid tumors-interim results. Mol Cancer Ther 2011;10:Suppl 11
- Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol 2010;7:209-19
- Petroulakis E, Mamane Y, Le Bacquer O, et al. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer 2005;94:195-9
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9-22
- Chresta CM, Davies BR, Hickson I, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 2010;70:288-98
- Naing A, Aghajanian C, Raymond E, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer 2012;107:1093-9
- Shimizu T, Tolcher AW, Papadopoulos KP, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res 2012;18:2316-25
- Carracedo A, Pandolfi P. The PTEN–PI3K pathway: of feedbacks and cross-talks. Oncogene 2008;27:5527-41
- LoPiccolo J, Blumenthal GM, Bernstein WB, et al. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist updat 2008;11:32
- She QB, Solit DB, Ye Q, et al. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 2005;8:287-97
- Wee S, Jagani Z, Xiang KX, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res 2009;69:4286-93
- Pal SK, Reckamp K, Yu H, et al. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs 2010;19:1355-66
- Apontes P, Leontieva OV, Demidenko ZN, et al. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011;2:222-33
- Zakikhani M, Blouin MJ, Piura E, et al. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat 2010;123:271-9
- Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011;71:4366-72
- Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol 2009;27:3271-3
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis 2011;2:e199
- Marks P, Rifkind RA, Richon VM, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001;1:194-202
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006;5:769-84
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 2009;27:5459-68
- Kawamata N, Chen J, Koeffler HP. Suberoylanilide hydroxamic acid (SAHA; vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood 2007;110:2667-73
- Gupta M, Ansell SM, Novak AJ, et al. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood 2009;114:2926-35
- Kodani M, Igishi T, Matsumoto S, et al. Suppression of phosphatidylinositol 3-kinase/Akt signaling pathway is a determinant of the sensitivity to a novel histone deacetylase inhibitor, FK228, in lung adenocarcinoma cells. Oncol Rep 2005;13:477
- Mahalingam D, Medina EC, Esquivel JA II, et al. Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels. Clin Cancer Res 2010;16:141-53
- Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res 2006;66:5549-54
- Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 2004;10:594-601
- Chaturvedi D, Gao X, Cohen MS, et al. Rapamycin induces transactivation of the EGFR and increases cell survival. Oncogene 2009;28:1187-96
- Weihua Z, Tsan R, Huang WC, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008;13:385-93
- Engelman JA, Cantley LC. A sweet new role for EGFR in cancer. Cancer Cell 2008;13:375-6
- Takeuchi H, Kondo Y, Fujiwara K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 2005;65:3336-46
- Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther 2005;4:101-12
- Fan Q-W, Specht KM, Zhang C, et al. Combinatorial efficacy achieved through two-point blockade within a signaling pathway—a chemical genetic approach. Cancer Res 2003;63:8930-8
- Li B, Chang C-M, Yuan M, et al. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res 2003;63:7443-50