Abstract
Introduction: Allergic rhinoconjunctivitis is an increasingly common source of morbidity with sensitivity to cats accounting for 10 – 15% of the disease burden. Allergy to cats is a major risk factor for the development of asthma.
Areas covered: Within the present manuscript, the current data on a novel therapeutic approach to treat cat allergy is reviewed. Cat Peptide Antigen Desensitisation (Cat-PAD) is a mixture of seven small peptides developed for the treatment of cat allergy. It is designed to induce immunological tolerance via binding to MHC class II on antigen presenting cells and interacting with regulatory T cells without triggering the cross-linking of IgE on mast cells and basophils. The peptide sequences are derived from the major cat allergen Fel d 1. The peptides have been selected to ensure a similar T cell response to that generated to whole cat dander in ex-vivo PBMC derived from cat allergic individuals. The size of the peptides is insufficient to induce cross-linking of IgE. Clinical data from a series of studies shows that Cat-PAD is able to significantly reduce allergic rhinoconjunctivitis symptoms after a short course of four injections over 12 weeks, and that the treatment effect is persistent lasting 2 years after the start of treatment.
Expert opinion: Taken together Cat-PAD is a novel, well tolerated and promising therapeutic approach to treat cat allergic patients. Data from the current international Phase III study will unravel whether the concept is also efficient and tolerable under daily life circumstances.
1. Introduction
Allergic rhinitis affects approximately 25% of the population of westernized countries Citation[1] and is a major cause of morbidity with reduced quality-of-life and impaired work/school performance. Cat dander is a major allergen source affecting 10 – 15% of subjects with rhinitis and is a major risk factor for asthma Citation[2,3]. Cat dander is pervasive making complete avoidance impossible Citation[4]. Although symptomatic treatment with nasal corticosteroids and antihistamines are a first-line of defence for many, there remain a substantial number of patients (40% in one series) who fail to respond to these drugs Citation[5].
The only curative treatment in IgE-dependent allergic disease is specific immunotherapy (WAO) Citation[6]. The allergen is given at increasing concentrations by different routes to affected patients. It has been shown that the treatment is not only efficacious, but also induces profound immunological changes promoting tolerance Citation[7,8].
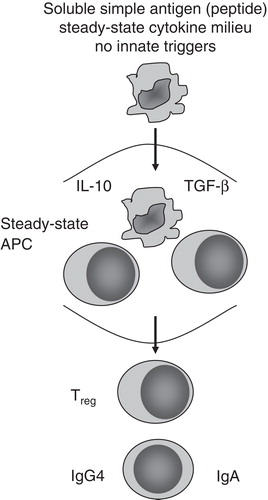
A central mechanism of immunotherapy is the induction of long-term tolerance. Synthetic peptides comprised of T cell epitopes whose sequence is derived from known allergen amino acid sequences employ a novel strategy to induce tolerance (). These short linear peptides are manufactured and retain the ability to stimulate antigen-specific T cell responses while being of insufficient length to cross link IgE on the surface of mast cells and basophils. Studies have provided preliminary evidence of efficacy in subjects allergic to bee venom Citation[9] and cats Citation[10]. In a series of studies using a mixture of 11 – 12 peptides it has been demonstrated that administration of Fel d 1 derived peptides resulted in reductions of surrogate endpoints including early- and late-phase skin responses intradermal injection of whole allergen Citation[11], together with nasal symptoms Citation[12] and airway hyper-reactivity Citation[13]. Mechanistic studies indicated treatment with peptides suppressed T cell proliferation and production of IL-4, IL-13 and IFN-γ ex vivo in the presence of recombinant Fel d 1, whereas IL-10 production was enhanced Citation[11,14].
2. Background
Through the years, various chemical modifications of allergens have been attempted to enhance efficacy, improve safety and foster compliance with AIT. A major goal in these efforts has been to develop a therapeutic construct in which the efficacy: safety benefit ratio is enhanced, preferably through heightened or maintained immunogenicity paralleled by reduced allergenicity Citation[15-17].
One such approach that offers the potential to suppress IgE-dependent diseases through induction of tolerance was that identified in studies carried out in the 1990s by Gefter and colleagues. Gefter developed synthetic T cell tolerizing peptides (15 – 22 mers) of cat Fel d 1 and ragweed Amb a 1 after the primary structure of the antigen had been determined by T.P. King Citation[18,19].
In collaborative studies in cat-allergic Citation[20,21] and ragweed-allergic Citation[22,23] research subjects, it has been shown that peptide immunization significantly reduced clinical symptoms without an increased antibody response.
Although, the clinical development program of this first peptide generation has never been completed a new class of Synthetic Peptide Immuno-Regulatory Epitopes (SPIREs) for treatment of allergic disease has been developed. Cat-PAD is the first in this new class of SPIREs and the clinical studies, which have been conducted so far are highlighted in this article.
Box 1. Drug summary.
3. Cat-PAD
To date two clinical studies performed on Cat-PAD (also known as ToleroMune Cat) have been published, and the results from several other studies have been presented as abstracts and posters at conferences. It is important to note that Cat-PAD is differentiated from both the early studies conducted by Norman Citation[20,21] and the mixture of peptides tested in the studies performed by the Imperial College researchers Citation[11-14]. Norman Citation[20,21] utilised two 27 amino acid peptides (Cat-PAD: seven 13 – 17 amino acid peptides) administered sub-cutaneously (Cat-PAD intradermal) used much higher doses up to 750 µg of peptide (Cat-PAD 75 µg), and had different T cell epitopes present. These studies conducted used a mixture of 11 or 12 peptides (Cat-PAD 7 peptides), and did not include agents to prevent dimer formation despite peptides containing free cysteine residues (Cat-PAD is specifically formulated to prevent dimer formation).
The selection of peptides for inclusion in Cat-PAD was driven by MHC class II binding studies using appropriate cell lines and by conducting T cell proliferation and histamine release assays on ex vivo samples from cat allergic human volunteers Citation[24].
T cell proliferation assays identified a preferred peptide mixture of seven synthetic peptides which have the sequence CPAVKRDVDLFLT, EQVAQYKALPVVLENA, KALPVVLENARILNCV, RILKNCVDAKMTEEDKE, KENALSLLDKIYTSPL, TAMKKIQDCYVENGLI and SRVLDGLVMTTISSSK. Proliferative responses to cat dander allergen extract and Cat-PAD correlated closely indicating that the majority of T cell reactivity to cat dander can be accounted for by the epitopes contained within Cat-PAD Citation[24]. Cytokine responses to both Cat-PAD and whole cat dander were more frequent than proliferative responses, with greater than 90% of the panel showing an IL-10 response to Cat-PAD in vitro Citation[24]. Results from a different group using tetramer mapping identified many of the same epitopes that are included in Cat-PAD as dominant T cell epitopes in cat allergic individuals Citation[25] confirming the appropriateness of the epitope selection.
3.1 IgE-binding activity
Following the identification of T cell epitopes Cat-PAD was evaluated for its potential to cause IgE cross-linking employing a basophil histamine release assay using whole blood sourced from cat allergic individuals as a surrogate for tissue mast cells Citation[24]. The conduct of studies in whole blood provides assurance that any binding of the peptides to serum albumin or cell membranes that might recreate a three dimensional structure has been considered. Significant quantities of histamine release were seen with whole cat dander at concentrations as low as 10 ng/ml whereas histamine release in response to Cat-PAD at concentrations up to 10,000 ng/ml was < 5% of that caused by whole allergen. If the total dose of Cat-PAD currently being tested in Phase III trials were injected intravenously and distributed in a 5 L blood volume, the total concentration of peptides would be < 15 ng/ml. This suggests that Cat-PAD has significantly less potential than whole allergen to cross-link IgE responsible for both local injection site but also systemic reactions. The reduced risk of local and systemic reactions is also critical for the therapy regime as it allows a therapeutic dose of Cat-PAD to be given from the start of treatment, without the need for dose escalation. Cat-PAD is not adjuvanted. This may be important in ensuring the development of the product without lengthy delays due to regulatory concerns, particularly in the USA where the Food and Drug Administration has placed several adjuvanted products on clinical hold during their development.
3.2 Toxicology and safety pharmacology
A toxicology and safety pharmacology program to demonstrate the safety of Cat-PAD has been conducted. Doses of Cat-PAD well in excess of the intended clinical dosing regimen have been evaluated in single and repeat dose toxicology studies. No undesirable pharmacological activity was observed either in the toxicology studies (personal communication from Circassia) or in specific safety pharmacology studies evaluating cardiovascular, respiratory and neurological function. Reproductive toxicology studies and juvenile toxicology studies have now been completed and again no significant toxicological sequelae were observed (personal communication from Circassia).
3.3 Findings from animal models
One study has been conducted using one of the peptides (KALPVVLENARILKNCV) from Cat-PAD in a novel HLA-DR1 transgenic mouse model Citation[26]. Mice were sensitized with 10 μg recombinant Fel d 1 in alum on days 0 and 14, and challenged intra-nasally with 250 μg cat allergen extract on days 22, 23 and 24 to localise the response to the airway. A single intra-dermal injection of 1 μg of the peptide KALPVVLENARILKNCV or a DR1-binding control was administered on day 25 and mice were re-challenged with cat allergen on days 59 and 60. Following analysis of lung function on (day 61), the mice were sacrificed.
Results from the study demonstrated that treatment with the peptide found in Cat-PAD resulted in reductions in bronchoalveolar lavage total cells and eosinophils, reductions in pulmonary and systemic TH2 cytokines, reduced proliferative responses to Fel d 1, and reduced recruitment of TH2 cells to the lungs. Administration of an anti-IL-10 monoclonal antibody immediately after treatment with the peptide blocked these changes indicating that IL-10 is a critical cytokine in the mechanism of action of SPIREs to re-establish tolerance. While care must be taken when trying to relate findings in an acute sensitization animal model to the clinical setting, the demonstration of a critical role for IL-10 in this study is consistent with current understanding of the importance of this cytokine as a regulatory cytokine in humans; similarly consistent is the observation that the mixture of peptides in Cat-PAD induced IL-10 release from PBMC in vitro from greater than 90% of a tested panel of cat allergic subjects Citation[24].
3.4 Clinical development
First in human study indicated an excellent tolerability. The first-time-in-human study for Cat-PAD evaluated the safety of a single dose given intra-dermally (doses between 0.03 nmol and 12 nmol) or subcutaneously Citation[24]. The study evaluated the change in area of the late phase skin response following intra-dermal injection of whole allergen 1 week before and 3 weeks after a single injection of Cat-PAD given intra-dermally. Intra-dermal injection of Cat-PAD show consistent reductions in the area of the Late Phase Skin Response following whole allergen challenge. The safety findings were unremarkable and the development progressed into repeat administrations studies.
3.5 Clinical studies from the environmental exposure chamber
The subsequent clinical development of Cat-PAD has focused on the use of an environmental exposure chamber (EEC) model of cat allergy. The EEC allows the introduction of aeroallergens in a highly controlled manner with minimal variation in allergen exposure and the ability to utilize pre-established allergen levels known to induce symptoms at the moderate to severe level Citation[27,28].
One of the main difficulties in developing allergen immunotherapy products is the systematic evaluation of the effect of dose and dosing regimen on symptom scores.
3.6 First EEC study reveals a dose-dependent clinical efficacy
The first EEC study with Cat-PAD analyzed the relationship between dose of product, dosing regimen and symptom scores in cat allergic subjects following challenge with aerosolized cat allergen in an EEC Citation[29,30]. Subjects attended a baseline EEC visit consisting of 4 consecutive days of 3 h in the chamber. One-hundred and twenty-one subjects who met the threshold symptom score were randomized to one of four treatment arms (4 administrations of 3 nmol 2 weeks apart, 4 administrations of 6 nmol 2 weeks apart, 4 administrations of 3 nmol 4 weeks apart, 8 administrations of 3 nmol 2 weeks apart or placebo). Following treatment with Cat-PAD or placebo subjects returned 18 – 22 weeks after the start of treatment for post-treatment challenge (PTC) consisting of a further 4 consecutive days of 3 h in the EEC.
The primary efficacy measurement was the TRSS a composite score comprising four nasal and four ocular symptoms. At all EEC visits subjects recorded their symptoms in a diary just before entering the EEC and every 30 min while in the chamber. Symptoms were divided into: nasal symptoms (running nose, sneezing, blocked nose, itchy nose) and ocular symptoms (itchy eyes, watery eyes, red eyes, sore eyes). For each symptom, the subject rated the severity as follows: 0 – absent, 1 – mild/barely noticeable, 2 – moderate/annoying/bothersome and 3 – severe/incapacitating. Subjects were required to have a TRSS of at least 10 out of 24 and nasal symptoms of at least 6 out of 12 on at least one time point on days 3 and 4 at baseline challenge. The primary endpoint was the difference in TRSS at each time point on each day between baseline and PTC Citation[29,30].
Frequencies of all TEAE (related and unrelated to treatment) in the active treatment arms were less than in the placebo cohort with the exception of the 6 nmol cohort which trended slightly higher. Analysis of the respiratory system TEAEs showed no evidence of any safety signal after treatment with Cat-PAD. Respiratory system TEAEs, including asthma, dyspnoea and wheezing, occurred at a low frequency in both active and placebo groups, with no obvious difference between the groups and with the significant majority due to cat allergen exposure in the EEC.
Treatment with Cat-SPIRE showed greater efficacy when dosed over 12 – 14 weeks than when dosed over 6 weeks. The eight administrations of the 3 nmol dose showed a statistically significant reduction in symptoms versus placebo (p < 0.05) in subjects who attended the main centre for all their visits. The 6 nmol dose showed a trend to be superior to the 3 nmol dose, albeit tested in a suboptimal regimen Citation[29,30].
As exemplified in for the 8 × 3 nmol group, the difference between Cat-PAD treatment and placebo increased as the cumulative dose of allergen rose over successive days. Greater improvements in clinical scores were observed in the Cat-PAD treated group on successive challenge, whereas scores for the placebo group remained largely unchanged. At time points from 1 h onwards on days 2, 3 and 4 in the EEC the mean difference between placebo and the 8 × 3 nmol 2 weeks apart regimen was 2.9 units on the TRSS scale.
Figure 2. Difference in Total Rhinoconjunctivitis Symptom Score (TRSS) at each 30 min time point (3 h per day) in the chamber over 4 consecutive days; score at Baseline Challenge minus score at Post Treatment Challenge (PTC), 8 × 3 nmol Cat PAD and Placebo.
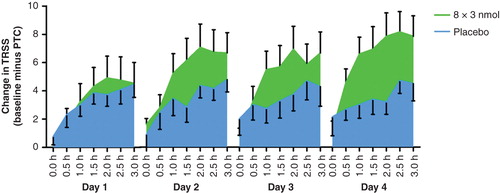
The primary efficacy variable was the change from baseline TRSS at the post-treatment visit using all time points in Cat-PAD treatment groups compared to placebo 17 – 21 weeks after the first treatment visit. plots the mean treatment effect at each time point on each day for subjects from the ITT population receiving 8 × 3 nmol Cat-SPIRE 2 weeks apart versus placebo. It is notable that the treatment effect appears greatest on days 2, 3 and 4 at time points after 1 h, suggesting that the treatment effect may either be enhanced by exposure to allergen or is greater when late phase reactions may be present (i.e., 24 h after allergen challenge).
3.7 Second EEC study suggests long-term efficacy
Following the results of the first exposure chamber study, a second study was conducted to compare the eight administrations of the (3 nmol dose) versus four administrations of a (6 nmol dose) given 4 weeks apart.
The main objective of this second study was to understand if combining the higher dose used in the first EEC study with a dosing regimen over a longer time period could outperform the best performing regimen from the previous study. The study was designed similarly, with the baseline challenge consisting of 4 consecutive days of 3 h in the exposure chamber. Following treatment subjects returned to the exposure chamber for 4 consecutive days of 3 h. Subjects who completed the study were enrolled in a further follow-on study which evaluated the treatment effect at 1-year, and again at 2-years.
As the goal of immunotherapy is to induce a long-lasting persistent treatment effect that is maintained after cessation of treatment, this clinical study was crucial to show the potential of the disease modifying effect of the drug. A product which can achieve 1 or 2 years persistent treatment effects after a short course over 3 – 6 months represents a disease modifier and is superior over conventional pharmacotherapy.
The results of this study Citation[31] indeed showed a persistent treatment effect as the treatment effect was apparent at 18 – 22 weeks and persisted at the 1 year exposure chamber visit. plots the treatment effect (difference in symptom scores between baseline and challenge at one year) for placebo and (4 × 6 nmol) Cat-PAD. It can be seen that the treatment effect is present on all 4 days in the chamber. Inspection of the absolute symptom scores on days 1 – 4, again showed a treatment effect that appeared to grow stronger with cumulative allergen challenge. The treatment effect at 1 year showed statistically significant improvements in total rhinoconjunctivitis symptom score of four units on the TRSS scale – and the magnitude of effect was larger than that observed at 18 – 22 weeks. Statistically significant improvements in nasal and ocular symptom scores were also reported, demonstrating Cat-PAD had a robust treatment effect on the two main organs affected by rhinoconjunctivitis.
Figure 3. Difference in Total Rhinoconjunctivitis Symptom Score (TRSS) at each 30 min time point (3 h per day) in the chamber over 4 consecutive days 1 year after the start of treatment; score at baseline challenge minus score at post Treatment challenge (PTC), 4 × 6 nmol Cat PAD and placebo.
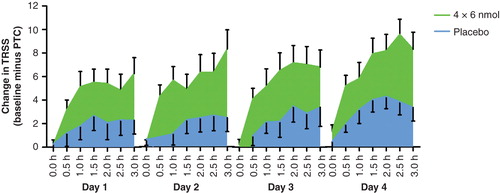
In the subsequent follow-up study, at 2 years, Citation[32], four administrations of a (6 nmol dose) 4 weeks apart showed a persistent treatment effect on TRSS compared to placebo. Post-hoc inspection of the nasal symptom scores showed statistically significant differences between the four administrations of 6 nmol and placebo at multiple time points during the 4 days of the exposure chamber challenge. In particular, there were highly statistically significant differences in nasal symptom scores on day 4 time points after 2 h, 2.30 h and 3 h in the exposure chamber when the cumulative allergen challenge is greatest Citation[33]. Nasal congestion, is a particular problem in perennial allergy. The demonstration of a sustained treatment effect 2 years after the start of treatment on the most difficult and intransigent component of rhinoconjunctivitis is highly encouraging.
4. Other approaches for cat allergy
4.1 Clinical comparative studies
Direct comparison of the efficacy of Cat-PAD with that of other interventions is complicated by differences in study design and mode of allergen exposure. Relatively few studies have been conducted specifically evaluating the treatment benefits of either pharmacotherapy or immunotherapy using cat dander as the allergen. summarizes the treatment effects seen for Cat-PAD and other immunotherapy/pharmacotherapy conducted specifically with cat allergen.
Table 1. Summary of clinical studies conducted with cat allergen.
4.2 SCIT with cat dander extract
A double-blind placebo controlled study employing a cat room design was conducted at a UK allergy centre Citation[34]. Specific immunotherapy was performed with a depot preparation of cat dander extract of declared potency (Alutard SQ, ALK, Denmark). This extract is immunochemically and biologically standardized and adsorbed onto aluminium hydroxide as an adjuvant. Subcutaneous injections were performed at a frequency of two injections per week with progressive dose escalation and reached a maintenance dose after approximately 3 months. Adjustments to the schedule were made on an individual basis as indicated by large local reactions (> 5 cm diameter) or mild systemic symptoms following the previous injection. The maintenance dose was 100,000 SQ units containing 15 µg Fel d 1. After reaching the maintenance dose subjects received a further injection after 2 weeks, and then every 4 weeks thereafter.
In this study, subjects attended a standardized cat challenge by visiting a house in which three cats had lived for over 8 years. Subjects scored their symptoms for the following categories: nasal (4), chest (3), ocular (2), throat irritation (1) – each on a scale of 0 – 3 with 0 being absent, 1 being mild, 2 moderate and 3 severe. Symptoms were scored every 5 min for 15 min, then every 15 min for 2 h and every hour for a further 5 h. (Thus symptoms were scored on a total of 15 occasions.) Symptom scores at each time point were added to give an overall cumulative score made up of 10 components × 15 measurements.
At baseline, subjects on active and placebo had mean scores of around 60, and adjusting this for the 15 time points measured yields an average Total Symptom Score (TSS) at each time point of approximately 4, a relatively modest score compared to the mean TRSS of 12 – 14 observed in the Cat-PAD exposure chamber studies. Following treatment with Cat immunotherapy, an improvement in the average TSS at each time point of approximately 0 units was reported for placebo and an improvement of 3 units was reported for subjects treated with Alutard. The lack of a placebo response in this study is a slightly unusual finding in studies of this nature. Indeed the studies with Cat-PAD at 1 and 2 years showed an improvement in TRSS of 7 – 8 units from baseline. This suggests the treatment effect of Cat-PAD 9 months and 21 months after the cessation of treatment is at least as good, and probably better, than the treatment effect achieved with whole allergen immunotherapy.
4.3 Data from sublingual clinical studies
The effects of sublingual cat allergy drops, on symptoms of cat allergy conducted using the same exposure chamber in Canada that has been used for the Cat-PAD studies has been published Citation[35]. Subjects were randomized to placebo or to one of three doses of sub-lingual cat allergen drops containing 4.5 µg, 9 µg or 18 µg of Fel d 1/day of ALK cat hair extract. The subjects were treated daily for 16 weeks and were exposed to cat allergen in the EEC at baseline and at 8 and 16 weeks after the start of treatment. The TSS was made up of four nasal and two ocular symptoms, each rated on a 4 point scale (0[none] – 3 [severe]). The changes from baseline TSS at 16 weeks were: 0.5 TSS units (4.5 µg Fel d 1), 1.36 TSS units (9 µg Fel d 1), 3.22 TSS units (18 µg Fel d 1); 1.68 TSS units (Placebo). Interestingly the two lower dose groups performed less well than placebo, while the treatment effect with the highest dose versus placebo is approximately 1.5 units. The treatment effect for sublingual cat allergen drops after daily treatment for 16 weeks appears to be substantially less than the treatment effect of Cat-PAD at both 9 and 21 months after a course of 4 injections over 12 weeks. Thus, overall, in the context of other studies employing similar study designs or means of evaluation, a short course of Cat-PAD compares favourably with existing pharmacotherapy and immunotherapy, and indirect comparison suggests Cat-PAD may provide a superior improvement in symptom indices when contrasted to the findings in studies which compared either SCIT or pharmacotherapy to placebo.
4.4 Data from intralymphatic IT for cat allergy
The intralymphatic immunotherapy (ILIT) is novel route of specific immunotherapy were allergen is directly delivered to B- and T-cells Citation[36]. In randomized controlled Phase I/II clinical trials in patients with grass pollen induced rhinoconjuctivitis 3 intralymphatic injections with grass pollen allergen extract induced nasal tolerance Citation[37]. The same approach has been reported in cat allergic patients, where a modular antigen transporter (MAT)-Fel d 1 recombinant non-glycosylated fusion protein consisting 191 amino acid was used. Twelve patients were treated with verum and eight with placebo Citation[38]. The data from this small clinical trial indicated after three injections a 74-fold increase in nasal tolerance. A follow-up visit after 300 days revealed less symptoms in the MAT-Fel d 1 group, however, no baseline values were assessed. The treatment was well tolerated and in the verum group no severe adverse events occurred.
4.5 Pharmaco-based symptomatic treatment
A study of the efficacy of the antihistamine fexofenadine under conditions of cat allergen exposure, has also been reported Citation[39]. Cat allergen exposure took place in a challenge room which shared the same ventilation system as a cat shelter housing up to 80 cats. Patients remained in the room until they could no longer tolerate their symptoms: up to 30 min at a baseline visit and 60 min at the treatment visits. Cat bedding (towels) was shaken vigorously before subject entry and once every 15 min while the subjects were in the cat challenge room.
The TSS was defined as the sum of the following symptoms: rhinorrhea (nasal discharge/runny nose or postnasal drip), itchy nose/palate/throat, sneezing and itchy/watery/red eyes using a 5-point scale from 0 (absence of symptoms) to 4 (severe symptoms that were very bothersome). Nasal congestion was also measured by the patients' evaluation of their symptoms and based on a 5-point scale of nasal stuffiness from 0 (clear, fully open) to 4 (blocked, with no air movement through the nostrils). Subjects scored their symptoms every 5 min during the challenge.
The study followed a cross-over design, with study drug being administered prophylactically. Subjects were randomly assigned to receive fexofenadine (180 mg) or placebo 90 min before entry into the challenge room. The two treatment periods were separated by a wash-out of 14 days. The primary efficacy end point was the change from baseline (pre-dose) in the TSS 30 min after initiation of the cat allergen challenge (2 h after dosing) and showed a difference in the mean change from baseline TSS of 1.3 units between fexofendadine and placebo. The use of fexofenadine prophylactically in this model is unusual and enables systemic concentrations to be achieved in advance of exposure to allergen. Nevertheless the treatment effect of this anti-histamine against cat dander (1.3 units on TSS scale) is modest when compared to the treatment effect of Cat-PAD 9 and 21 months after the cessation of treatment.
5. Safety profile of Cat-PAD
The clinical data for Cat-PAD is complemented by an encouraging set of safety data. A summary of the safety data generated in the clinical studies conducted to date is shown in and shows so far no evidence for any IgE-mediated systemic reactions or anaphylactic reactions Citation[40]. Two serious adverse events have been reported (skin laceration – placebo; headache – 3 nmol Cat-PAD). The incidence of the most commonly reported adverse events was comparable between Cat-PAD and placebo: headache (Cat-PAD 15.6%; placebo 18.2%), upper respiratory tract infection (Cat-PAD 15.6%; placebo 12.6%), bronchospasm (Cat-PAD 8.1%; placebo 8.4%) and cough (Cat-PAD 4.7%; placebo 4.9%); bronchospasm was mostly associated with allergen challenge in the EEC. Importantly the incidence of adverse events leading to discontinuation is comparable between Cat-PAD and placebo groups ().
Table 2. Four Phase II prospective, randomized, double-blind, placebo-controlled studies are summarized.
In the integrated safety database, to date, only two injection site reactions have been reported as adverse events: one each following 3 nmol Cat-PAD and placebo. Injection sites were reported as abnormal but not clinically significant at one or more assessments in 7.4, 4.4 and 4.7% of patients following 3 nmol, 6 nmol Cat-PAD and placebo, respectively Citation[41].
6. Conclusion
In the present review the available data for Cat-PAD, the first in a new class of SPIREs, has been summarised. The available data from the first clinical trials is promising regarding both efficacy and tolerability. Cat-PAD contains seven synthetic, short, peptides derived from the major cat allergen Fel d 1 and is given intradermally. This novel product, when compared to existing whole allergen immunotherapy, advances the ability to immunize patients considering three critical differentiating parameters:
The allergenic source -- by using synthetic short peptides with a known amino acid sequence, which are chemically synthesised and assayed using standard pharmaceutical techniques such as liquid chromatography, it is possible to avoid any variability in the potency of product from vial to vial and batch to batch. [Variability in the potency of allergen from batch to batch is a recognized problem with existing immunotherapy products.]
The route of administration by intradermal application of allergens can promote definitive immunotolerance through regulatory T cell induction.
The Cat-PAD product does not provoke IgE-mediated local and systemic reactions. This allows a therapeutic dose to be administered from the outset of treatment. A 6 nmol dose of Cat-PAD contains approximately 75 µg of peptides, representing a 5 fold excess over the target maintenance dose (15 µg Fel d 1) for specific immunotherapy.
The use of a higher dose when compared to conventional immunotherapy, the lack of expression of pro-inflammatory co-stimulatory molecules on antigen presenting cells and the intradermal route of administration are all likely important contributors to the high levels of efficacy seen with Cat-PAD after just 4 injections over a 12 week period.
The data obtained so far for Cat-PAD need to be confirmed in large scale controlled clinical studies in a real life setting. In the case of cat allergy this requires evaluating the clinical efficacy and tolerability in patients who are keeping a cat in their house. Presently subcutaneous specific immunotherapy with cat allergen extracts is associated with a high risk of severe reactions and in Germany, therefore, is only presently performed in exceptional patient groups, for example personnel handling animals routinely as part of their job, or veterinarians.
The difficulty in conducting immunotherapy in cat allergy because of the high incidence of adverse events may explain the lack of other therapeutic options in development specifically for treating cat allergy. While both specific immunotherapy and sublingual immunotherapy as well as antihistamines are utilised for the treatment of cat allergy in clinical practice, the available data () generated in similarly designed studies performed in either an exposure chamber or a cat room shows the treatment effect of Cat-PAD to be comparable, if not superior, to that observed in clinical trials with these alternative treatment modalities.
7. Expert opinion
Taken together, collective data for Cat-PAD is promising, and provided the Phase III clinical data demonstrate similar levels of clinical efficacy and tolerability to those seen in the Phase II studies conducted to date, Cat-PAD may provide the allergist with an important step-change in the available therapeutic options for treatment of cat allergy in the future.
Declaration of interest
M Worm has been the PI of the Phase II clinical study, sponsored by Circassia and has also received a consulting honoraria from Circassia. PS Creticos has received grant support from Circassia.
Notes
Bibliography
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004;24:758-64
- Arbes SJ Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 2007;120:1139-45
- Bousquet PJ, Leynaert B, Neukirch F, et al. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy 2008;63:1301-9
- Morris DO. Human allergy to environmental pet danders: a public health perspective. Vet Dermatol 2010;21:441-9
- Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev 2003;4:CD001186
- Canonica GW, Bousquet J, Casale T, et al. Sublingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy 2009;64(Suppl 91):1-59
- Nouri-Aria KT, Durham SR. Regulatory T cells and allergic disease. Inflamm Allergy Drug Targets 2008;7(4):237-52
- Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol 2009;123(4):735-46
- Muller U, Akdis CA, Fricker M, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol 1998;101:747-54
- Norman PS, Ohman JL, Long AA, et al. Treatment of cat allergy with T-cell rective peptides. Am J Respir Crit Care Med 1996;154:1623-8
- Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet 2002;360:47-53
- Alexander C, Ying S, Kay AB, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy 2005;35:52-8
- Alexander C, Tarzi M, Larché M, Kay AB. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy 2005;60:1269-74
- Oldfield WL, Kay AB, Larché M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol 2001;167:1734-9
- Broide DH. Immunomodulation of allergic disease. Annu Rev Med 2009;60:279-9
- Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. Annu Rev Immunol 2010;28:211-41
- Focke M, Swoboda I, Marth K, et al. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy 2010;40:385-97
- Briner TJ, Kuo MC, Keating KM, Rogers BL. Peripheral T-cell tolerance induced in naive and primed mice by subcutaneous injection of peptides from the major cat allergen Fel d I. Proc Natl Acad Sci USA 1993;90:7608-12
- Wallner BP, Gefter ML. Immunotherapy with T-cell-reactive peptides derived from allergens. Allergy 1994;49:302-8
- Norman PS, Ohman JL Jr, Long AA, et al. Treatment of cat allergy with T cell epitope containing peptides. Am J. Respir Crit Care Med 1996;154:1623-8
- Norman PS, Nicodemus CF, Creticos PS, et al. Clinical and Immunologic Effects of Component Peptides in Allervax® Cat. Int Arch Allergy Immunol 1997;113:1-3
- Creticos PS, Hebert J, Philip G; The Allervax® Ragweed Study Group. Efficacy of Allervax® ragweed in the treatment of ragweed-induced allergy. J Allergy Clin Immunol 1997;99(1 Pt 2):S401; 1631
- Creticos PS. Peptide Downregulation of the Immune Response. In: Marone G, Austen KF, Holgate ST, Barry Kay A, Lichtenstein LM, editors. Asthma and allergic diseases. Academic Press, Elsevier Ltd; 1998;30:407-15
- Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol 2011;127:89-97
- Kwok WW, Roti M, Delong JH, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol 2010;125:1407-9
- Campbell JD, Buckland KF, McMillan SJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med 2009;206:1535-47
- Day JH, Horak F, Briscoe MP, et al. The role of allergen challenge chambers in the evaluation of anti-allergic medication: an international consensus paper. Clin Exp Allergy Rev 2006;6:31-59
- Salapatek AM, Patel P, Chan K, et al. An environmental exposure chamber -specific rhinoconjunctivitis quality of life questionnaire: the symptoms of seasonal allergic rhinitis correlate with the quality of life of patients with ragweed allergy in the chamber. Allergy 2007;62:118-19
- Larché M, Patel D, Patel P, et al. Safety and efficacy of Fel d 1 derived peptide immunotherapy in a double-blind, placebo-controlled environmental exposure chamber (EEC) study. Allergy 2012;67(Suppl 96):1-97
- Hafner R, Patel P, Salapatek AM, et al. FEL d 1 peptide antigen desensitization safety and efficacy in a double-blind, placebo-controlled environmental exposure chamber study. World Allergy Organ J 2013;6(Suppl 1):P150
- Patel D, Couroux P, Hickey P, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol 2013;131:103-9
- Hafner R, Couroux P, Armstrong K, et al. Two Year Persistent Treatment Effect Achieved After 4 Doses of Cat-Peptide Antigen Desensitization (Cat-PAD) in an Environmental Exposure Chamber (EEC) Model of Cat Allergy. J Allergy Clin Immunol 2013;131:AB147
- Hafner RP, Couroux P, Armstrong K, et al. Total Nasal Symptom Scores are reduced in an EEC model of cat allergy two years after administration of 4 doses of Cat-PAD. Available from: http://www.sessionplan.com/EAACI-WAO2013/Abstract 1887
- Varney VA, Edwards J, Tabbah K, et al. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy 1997;27:860-7
- Patel D, Bernstein DL, Plunkett G, et al. Tolerability and efficacy of sublingual immunotherapy in cat allergic subjects studies in an environmental exposure chamber. Ann Allergy Asthma Immunol 2011;107:A105
- Senti G, Johansen P, Kündig TM. Intralymphatic immunotherapy: from the rationale to human applications. Curr Top Microbiol Immunol 2011;820:71-84
- Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A 2008;105:17908-12
- Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol 2012;129:1290-6
- Berkowitz RB, Braker S, Lutz C, et al. Efficacy of fexofenadine in the prophylactic control of cat allergen-induced allergic rhinitis. Ann Allergy Asthma Immunol 2006;96:327-33
- Haumann B, Powell J, Hafner R. Safety and Tolerability of Fel d 1-Derived Peptide Antigen Desensitization. J Allergy Clin Immunol 2013;131:AB38
- Powell J, Haumann B, Hafner RP. Cat-PAD, the first in a new class of synthetic peptide immuno-regulatory epitopes, demonstrates a favourable tolerability profile in patients with cat-induced rhinoconjunctivitis. Available from: http://www.sessionplan.com/EAACI-WAO2013/Abstract 220