Abstract
Introduction: Cardiovascular diseases (CVDs) are the number one cause of death globally. The dramatically high rate of cardiovascular morbidity and mortality has attracted wide concern and great attention within the pharmaceutical industry. However, ∼ 10,000 compounds are tested for every one drug that reaches the market. For this reason, it is helpful to recapitulate previous failures and learn from these experiences.
Areas covered: This paper focuses on the 10 cardiovascular drugs discontinued after reaching animal studies or Phase I – II clinical trials between 1 January 2013 and 31 December 2014.
Expert opinion: The trend of increasing numbers of cardiovascular drug development terminations seen in recent years has changed. Only 10 cardiovascular drugs were discontinued after reaching animal studies or Phase I – II clinical trials between 2013 and 2014. Only two candidates were discontinued in the Phase I clinical evaluation, and eight were discontinued during Phase II development. Most discontinuations were attributed to lack of efficacy and safety. One orphan drug (RTA-402) appeared in the list of discontinued cardiovascular drugs. The most eye-catching one of the 10 discontinued drugs is RG-7652, a monoclonal antibody against PCSK9, which is predicted as the next statin.
1. Introduction
As the number one cause of death globally, cardiovascular diseases (CVDs) are a group of disorders of the heart and blood vessels, which include coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease and deep vein thrombosis and pulmonary embolism Citation[1]. It is estimated that > 23 million people will die annually from CVDs by 2030 Citation[2]. The dramatically high rate of cardiovascular morbidity and mortality has attracted wide concern and great attention of pharmaceutical industry. It was predicted that the global cardiovascular therapeutic drug market would reach $139.8 billion in 2015 Citation[3]. However, ∼ 10,000 compounds are tested for an individual drug to eventually reach the market. It might be helpful recapitulating previous failures, reporting the reason for discontinuation and giving some meaningful advice. This paper focuses on the cardiovascular compounds discontinued after reaching animal studies or Phase I – II clinical trials between 2013 and 2014. Information for this perspective is mainly derived from search of Pharmaprojects.
2. Discontinued drugs
2.1 General overview
provides an overview of the 10 cardiovascular drug candidates removed from the CVD development pipeline between 1 January 2013 and 31 December 2014. Among these drugs, two candidates were discontinued in Phase I clinical evaluation. Eight were discontinued during Phase II development. The detailed information on the 10 drugs is given below.
Table 1. Cardiovascular drugs discontinued in 2013 and 2014.
2.2 Discontinued drugs in Phase I
2.2.1 DSP-9599
DSP-9599 is an oral direct renin inhibitor developed by Sumitomo Dainippon Pharma for treatment of hypertension. It decreases plasma renin activity and inhibits the production of angiotensin I, and all downstream angiotensin peptides in the renin–angiotensin system such as angiotensin II. A preclinical study showed that DSP-9599 (10 mg/kg) resulted in a higher survival rate of 100%, compared with 67% for aliskiren (30 mg/kg) in transgenic rats overexpressing both human renin and angiotensinogen genes. DSP-9599 had a superior renoprotective effect compared with aliskiren Citation[4]. In sodium-depleted monkeys, DSP-9599 reduced blood pressure as well as plasma renin activity. Data from a single-center, double-blind, randomized, placebo-controlled, single ascending-dose Phase I trial in healthy male volunteers (n = 68) demonstrated that DSP-9599 was well tolerated, with no serious adverse events reported. Pharmacokinetics was dose proportional, and a 160 mg dose of the drug had higher and longer lasting renin inhibitory activity than aliskiren Citation[5]. In March 2012, Sumitomo Dainippon Pharma listed the compound as being in Phase I for the treatment of hypertension Citation[6]. However, by January 2013, Dainippon Sumitomo Pharma had discontinued the development of DSP-9599 Citation[7].
2.2.2 R-118
The novel small-molecule R-118 activates the critical cellular energy sensor 5’-AMP-activated protein kinase (AMPK) via modulation of mitochondrial complex I activity. Activation of AMPK results in both acute responses and chronic adaptations, which serve to restore energy homeostasis. R-118 treatment resulted in increased glycolysis and lipolysis in skeletal muscle Citation[8]. Rigel previously investigated R-118 for the potential treatment of intermittent claudication (IC) Citation[9]. In 2014, the product entered Phase I clinical trial Citation[10]. However, R-118 was discontinued in August 2014 due to side-effects observed. Rigel also developed other AMPK activators, including R-419 and RGL, for the potential treatment of IC, diabetes, improving muscle endurance in chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF). In August 2014, development was ongoing Citation[11].
2.3 Discontinued drugs in Phase II
2.3.1 Limaprost alfadex
Limaprost alfadex is an oral prostaglandin E1 analog. Prostaglandins act on a variety of cells such as vascular smooth muscle cells causing constriction or dilation, on platelets causing aggregation or disaggregation and on spinal neurons causing pain. Prostaglandins have a wide variety of actions, including, but not limited to muscular constriction and mediation of inflammation Citation[12]. Limaprost alfadex has been shown to improve peripheral circulatory failure with a vasodilator action and an antithrombotic effect. It also improves poor blood flow in the nerve tissue in cervical spondylosis and normalizes nerve function. Limaprost alfadex was discovered from collaborative research between Ono Pharmaceutical (Ono) and Dainippon Sumitomo Pharma (DSP). It was approved for the treatment of ischemic symptoms such as skin ulcer, pain and coldness accompanying thromboangiitis obliterans in 1988; and for the treatment of subjective symptoms such as pain and numbness in the lower leg and walking disability associated with acquired lumbar spinal canal stenosis as an additional indication in 2001. The drug has been sold under the trade name of Opalmon® Tablets by Ono and Prorenal® Tablets by DSP. In 2011, Ono and DSP initiated Phase II clinical trials in Japan for the treatment of carpal tunnel syndrome Citation[13]. In 2013, these trials were discontinued because the study failed to demonstrate efficacy Citation[14]. Ono and DSP also discontinued the development of limaprost alfadex for the additional indication of cervical spondylosis in 2008 due to the failure to demonstrate the anticipated efficacy in a Phase II study in patients with the disease Citation[15]. However, it was verified by Seoul National University Hospital in November of 2014 that the study on the efficacy of oral limaprost alfadex after surgery for cervical myelopathy was still ongoing Citation[16].
2.3.2 Parogrelil hydrochloride
As an orally active inhibitor of phosphodiesterase and thromboxane A2 synthetase, parogrelil hydrochloride was developed by Taisho Pharmaceuticals and Nissan Chemical for the treatment of asthma and for the treatment of IC caused by atheriosclerosis obliterans Citation[17]. Study results proved that parogrelil hydrochloride improved the physical component and physical functioning scores of the Medical Outcomes Study 36-Item Short Form and the walking distance and stair climbing domains of the Walking Impairment Questionnaire Citation[18] and suggested that parogrelil hydrochloride could be expected to have therapeutic advantage for IC Citation[19]. The development of parogrelil hydrochloride had reached Phase II clinical trials Citation[20]; however, Taisho Pharmaceuticals and Nissan Chemical discontinued the study in 2014 as a result of a comprehensive review of the clinical trial results and knowledge obtained to date Citation[21]. Taisho and Nissan had been evaluating the potential of the drug for the treatment of IC caused by spinal canal stenosis. In August 2009, this development was discontinued following a comprehensive evaluation of clinical trial results Citation[22]. Indigo Pharmaceuticals also conducted Phase III trials for the reduction of symptoms of IC in patients with peripheral arterial disease, but no further development was reported.
2.3.3 RTA-402
RTA-402 is a triterpenoid anti-inflammatory agent. It has demonstrated a wide variety of potentially therapeutic mechanisms, including inhibition of inducible nitric oxide synthase and cyclooxygenase expression, stimulation of expression of cytoprotective enzymes such as NAD(P)H quinine oxidoreductase and hemeoxygenase-1, and reduction in pSTAT3 levels. It also activates the Nrf2, which controls the production of > 250 antioxidant and detoxification proteins. Activation of Nrf2 protects tissues by increasing cellular antioxidant content and suppressing inflammatory signaling pathways. It is known that chronic inflammation promotes type 2 diabetes and its complications, including cardiovascular disease and chronic kidney disease (CKD) Citation[23]. In 2010, RTA-402 was licensed to Kyowa Hakko Kirin by Reata Pharmaceuticals in China, Japan, Korea, Thailand and Southeast Asian countries for the treatment of CKD Citation[24]. Abbott acquired rights to develop and commercialize the drug outside USA, excluding certain Asian markets. In the Phase III placebo-controlled comparative clinical study in CKD patients with type 2 diabetes conducted by Reata in the USA, Europe, Canada, Australia, and Central America (the BEACON study), an increased cardiovascular event risk was observed, particularly heart failure, in patients in the RTA-402 arm of the study. Therefore, in November 2013 Kyowa Hakko Kirin decided to discontinue a suspended Phase 2 clinical study and to investigate a future development strategy for RTA-402 Citation[21]. On July 2 2014, Kyowa Hakko Kirin decided the future development direction in Japan for RTA-402 following a detailed analysis of the BEACON study data and consultations with the Pharmaceuticals and Medical Devices Agency (PMDA). The company decided to continue developing RTA-402 for CKD patients with type 2 diabetes with a particular emphasis on patient safety and planned to evaluate both the safety and efficacy of RTA-402 in a new Phase II clinical study, under the new direction Citation[25]. RTA-402 had been evaluated in Phase I studies for cancer Citation[26]. In 2008, orphan drug designation was assigned by the Food and Drug Administration (FDA) for the treatment of pancreatic cancer. However, no recent development was reported for this indication.
2.3.4 Dalcetrapib
As a cholesteryl ester transfer protein (CETP) inhibitor, which regulates lipids by lowering low-density lipoproteins (LDL) and increasing high-density lipoproteins (HDL) Citation[27], dalcetrapib was originally developed by Japan Tobacco for the treatment of dyslipidemia. In October 2004, Japan Tobacco and Roche established a licensing agreement for the late-stage development and commercialization of dalcetrapib. Under the terms of that agreement, Roche gained exclusive worldwide rights, excluding Japan and Korea, to develop and commercialize the compound. In 2006, the license was expanded to include Korea. This product had been developed by Roche for the treatment of dyslipidemia and for the prevention of cardiovascular events. On May 7 2012, following the results of the second interim analysis of the dalcetrapib dal-OUTCOMES Phase III trial, which evaluated the efficacy and safety profile of dalcetrapib when added to existing standard of care in patients with stable coronary heart disease (CHD) following an acute coronary syndrome (ACS), Roche decided to terminate the dal-OUTCOMES trial and all the studies in the dal-HEART programme. The results showed that dalcetrapib increased HDL cholesterol levels but had a minimal effect on LDL cholesterol levels. As compared with placebo, the median C-reactive protein level was 0.2 mg per liter higher and the mean systolic blood pressure was 0.6 mm Hg higher. Dalcetrapib did not alter the risk of the primary end point (cumulative event rate, 8.0% and 8.3%, respectively; hazard ratio with dalcetrapib, 1.04; 95% confidence interval, 0.93 to 1.16; p = 0.52) and did not have a significant effect on any component of the primary end point or total mortality Citation[28,29]. Phase II clinical trials in Japan conducted by Japan Tobacco had been underway for the treatment of dyslipidemia Citation[30]; however, Japan Tobacco discontinued the development of dalcetrapib in 2013 too Citation[31].
2.3.5 TC-5214
TC-5214 is the (S)-( + )-enantiomer of the racemate mecamylamine hydrochloride. It inhibits the activity of various neuronal nicotinic receptors (NNR) subtypes, including the alpha4beta2 NNR. In preclinical studies, TC-5214 displayed safety, pharmacology, pharmacokinetic and metabolic profiles appropriate for therapeutic development Citation[32]. At the same time, this compound exhibited superior potency compared with mecamylamine at specific NNR subtypes associated with depression Citation[33]. TC-5214 also inhibits LS (high calcium permeability low sensitivity) with greater efficacy than TC-5213 (the R-(-) stereoisomer of mecamylamine) and the compound significantly facilitated agonist-induced activation of HS (low calcium permeability high sensitivity). TC-5214 was more efficacious than TC-5213 in vivo, animal models of depression and anxiety Citation[34]. Preclinical data presented at the BIO 2003 meeting showed that TC-5214 demonstrated a rapid onset of action, with potency in animals, superior to that of the selective serotonin reuptake inhibitors (SSRIs) and the tricyclic compounds. TC-5214 had comparable efficacy to the SSRIs. The development of TC-5214 as augmentation therapy for major depressive disorder (MDD) had reached Phase III clinical trials Citation[35-39]. However, both AstraZeneca and Targacept discontinued these trials in 2012 since RENAISSANCE 4 and RENAISSANCE 5 did not meet the primary endpoint of change on the Montgomery-Asberg Depression Rating Scale (MADRS) total score after 8 weeks of adjunct treatment with TC-5214 as compared to placebo Citation[40]. Targacept had been conducting Phase II clinical studies for the treatment of overactive bladder aimed at salvaging this drug Citation[41,42]. In a Phase IIb clinical trial, the high dose of TC-5214 demonstrated mixed results on the co-primary endpoints by providing a statistically significant reduction in micturition frequency (p = 0.033) and an improvement that did not reach statistical significance on episodes of urinary incontinence (p = 0.379) per 24 h, after 12 weeks of treatment. As a consequence of these facts, Targacept finally discontinued the program in July 2014 Citation[43]. Targacept also previously developed the drug for hypertension. In August 2009, this trial was discontinued due to slow enrollment and the favorable results achieved in the MDD trial Citation[44].
2.3.6 NTC-801
NTC-801 is a selective inhibitor of the acetylcholine-activated potassium ion channel (IKACh). Because IKACh channel activity is apparent in the atria, not in the ventricles, NTC-801 could provide a more targeted and safer therapy for atrial fibrillation. This product had been developed by Teijin Pharma and Nissan Chemical Industries for the treatment of atrial fibrillation and flutter Citation[45]. In March 2009, Nissan Chemical Industries and Teijin Pharma granted Bristol-Myers Squibb (BMS) development and commercialization rights to NTC-801 in markets outside Japan. Under the terms of the collaboration agreement, NTC-801 was developed by BMS as an oral treatment for the maintenance of normal sinus rhythm in patients with atrial fibrillation Citation[46]. By 2011, NTC-801 had been under Phase II clinical development in Japan Citation[47]; however, in the first half of 2013, Nissan Chemical and Teijin Pharma discontinued the development of NTC-801 for the treatment of arrhythmia.
2.3.7 ISIS-CRPRx
ISIS-CRPRx is a generation 2.2 antisense drug that inhibits the C-reactive protein (CRP). CRP is strongly associated with the presence and severity of many diseases, including numerous inflammatory and cardiovascular diseases. It had been developed at Isis Pharmaceuticals for the treatment of inflammation, coronary artery disease, rheumatoid arthritis and paroxysmal atrial fibrillation. In August 2013, Isis Pharmaceuticals opted to throw in the towel on its rheumatoid arthritis program for ISIS-CRPRx after a Phase II study failed to produce a statistically significant improvement over a placebo, although ISIS-CRPRx cut levels of the inflammatory C-reactive protein by up to 67% for the high dose in that trial. Meanwhile, Isis Pharmaceuticals do plan to continue to evaluate ISIS-CRPRx to treat other diseases Citation[48].
2.3.8 RG-7652
RG-7652 is a monoclonal antibody directed against proprotein convertase subtilisin/kexin type 9 (PCSK9), a secreted protein that increases levels of low-density lipoprotein cholesterol (LDL-C) in the blood by promoting the degradation of LDL receptors in the liver. Inhibition of PCSK9 decreases circulating LDL-C, thereby potentially improving CV outcomes. As its mode of action differs from that of statins, anti-PCSK9 may provide benefit to people who do not achieve desirable LDL-C levels with statins or cannot tolerate them Citation[49].
RG-7652 was originally developed at Genentech, which is acquired by Roche in 2009, for the potential sc treatment of metabolic diseases, including hypercholesterolemia in patients with, or at high risk of, coronary heart disease. By July 2013, a Phase II study of the safety and efficacy of RG-7652 in patients with coronary heart disease or high risk of coronary heart disease had been completed Citation[50]. In September 2014, data were presented at the ESC 2014 Annual Congress in Barcelona, Spain. RG-7652 therapy was well tolerated and significantly and dose-dependently reduced LDL-c in all the arms with mean LDL-c reductions of 48 to 60% from baseline to nadir. However, in July 2014, Roche listed the drug as being discontinued from development for unknown reasons Citation[51].
3. Conclusion
Ten new drug candidates for the treatment of CVDs have been discontinued between 2013 and 2014. Eight compounds were discontinued during Phase II clinical trials (Limaprost alfadex, Parogrelil hydrochloride, RTA-402, Dalcetrapib, TC-5214, NTC-801, ISIS-CRPRx, RG-7652) and two compounds were discontinued during Phase I clinical trials (DSP-9599 and R-118). To be sure, the reason of discontinuation was not fully disclosed in most cases.
4. Expert opinion
The analysis of cardiovascular drugs discontinued in 2013 and 2014 reveals that the trend of increasing numbers of cardiovascular candidate drug development terminations seen in past years has changed. These 10 drugs discontinued after reaching animal studies or Phase I – II clinical trials, together with the other 5 drugs (ABH-001, FXV-673, MK-0431, Ixmyelocel-T and Edarbyclor) discontinued in Phase III clinical trials were the cardiovascular drugs dropped from the global cardiovascular drug development pipeline in the past 2 years. It was significantly fewer than the discontinuations reported in the past 6 years Citation[52-56]. shows the distributions of the number of the discontinued cardiovascular drugs in the past 8 years.
Figure 1. The distributions of the number of the discontinued cardiovascular drugs in the past 8 years.
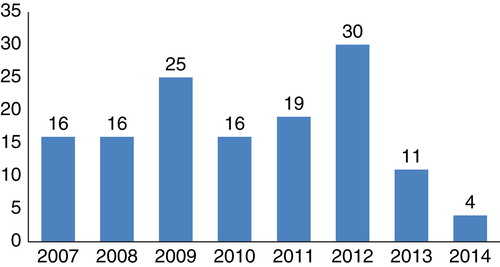
Among the failed cardiovascular drugs between 2013 and 2014, eight are eliminated in Phase II clinical trials and two in Phase I stage. The majority of these failures are because of efficacy and safety (six drugs), whereas one because of strategic factor, three others because of unspecified or unknown reasons. It shows that whether the Phase II clinical trial can achieve the desired goal is still the bottleneck in drug development. In general, failures due to lack of efficacy and safety demonstrate the need for the development of more predictive animal models where possible, the need to eliminate compounds that have mechanism-based toxicity and, more significantly, the need to develop experimental medicine paradigms that are more predictive of outcomes and to carry out such proof-of-concept clinical trials much earlier in development, especially during first-in-man studies. These approaches are likely to be valuable Citation[57].
The orphan drug development process is highly expensive and with the highest risk levels. One orphan drug (RTA-402) appeared in the list of discontinued cardiovascular drugs between 2013 and 2104. As an oral, pro-apoptotic, anti-inflammatory and antiangiogenic synthetic triterpenoid IKK, STAT3 and NFkB inhibitor and activator of NFE2L2 (Nrf2), orphan drug designation was assigned by the FDA to RTA-402 for the treatment of pancreatic cancer. It is a pity that the development of RTA-402 for IC was terminated and no recent development has been reported for cancer.
The most eye-catching one of the 10 discontinued drugs is RG-7652, a monoclonal antibody against PCSK9. Statins have been approved for the treatment of dyslipidemia for 28 years. This class has demonstrated substantial and consistent reduction of cardiovascular events with an acceptable safety profile. Despite the widespread use of statins, patients continue to experience residual risk. To date, attempts to reduce that risk with additional lipid therapies have failed. Trials of niacin and fenofibrate in subjects already on statins had shown no further risk reduction Citation[58,59]. As anti-PCSK9 may provide benefits to people who do not achieve desirable LDL-C levels with statins or cannot tolerate them, PCSK9 inhibitors attracted great attention of pharmaceutical industry Citation[60]. The PCSK9 inhibitors under development in 2014 were alirocumab (Sanofi/Regeneron) Citation[61], evolocumab (AMGEN) Citation[62], LGT209 (Novartis), 1D05-IgG2 (Merck), RG-7652 (Roche), LY3015014 (Eli Lilly) and bococizumab (Pfizer). On October 17 2014, Amgen sued Sanofi and Regeneron to prevent the allegedly infringing manufacture, use, and sale of Sanofi and Regeneron’s alirocumab Citation[63]. However, On January 26 2015, Sanofi and Regeneron Pharmaceuticals announced that the U.S. FDA had accepted for priority review the Biologics License Application (BLA) for Praluent™ (alirocumab) Citation[64]. On March 20 2015, Amgen announced that an application seeking marketing approval of RepathaTM (evolocumab) for the treatment of high cholesterol had been submitted for review to the Ministry of Health, Labour and Welfare in Japan Citation[65]. On the other hand, the development of RG-7652 was discontinued for unknown reasons, which is puzzling.
Overall, the range of the discontinued compounds discussed offers a relevant picture of what is currently in the pipeline for the CVD conditions such as those mentioned in this retrospective. Future researches may benefit from these developments and investigators conducting similar studies may learn from these failures.
The trend of increasing numbers of cardiovascular drug development terminations in past years has changed.
10 cardiovascular drug candidates were discontinued after reaching animal studies or Phase I – II clinical trials between 2013 and 2014.
Eight discontinuations occurred during Phase II clinical development.
Most discontinuations were attributed to efficacy and safety.
One orphan drug (RTA-402) appears in the list of discontinued cardiovascular drugs between 2013 and 2104.
Roche discontinued the development of a PCSK9 inhibitor (RG-7652), which is predicted as the next statin.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
This box summarizes key points contained in the article.
Bibliography
- WHO. Cardiovascular diseases (CVDs). 2015. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/ [Last accessed 27 March 2015]
- WHO. Cardiovascular disease. 2015. Available from: http://www.who.int/cardiovascular_diseases/en/ [ Last accessed 27 March 2015]
- Okokok. Cardiovascular Therapeutic Drugs: Technologies and Global Markets. 2010. Available from: http://www.okokok.com.cn/Htmls/PE_Product/100805/84271.html [ Last accessed 27 March 2015]
- Mori M, Hirata T, Ikeno A, et al. DSP-9599, a novel direct renin inhibitor, reduces renal injury in double transgenic rats over-expressing both human renin and angiotensinogen genes[Adstract PP.42.353]. 23rd Eur Meet Hypertens; June 14-17, Milan
- Ikeno A, Mori M, Kawai H, et al. A first-in-human, phase 1 study of DSP-9599, a novel direct renin inhibitor, with translational assessment in high-renin monkey[Abstract PP.20.162]. 23rd Eur Meet Hypertens; June 14-17, Milan) 2013
- Dainippon Sumitomo Pharma. Supplementary Financial Data for the Year Ended March 31, 2012. 2012. Available from: http://www.ds-pharma.com/ir/library/financial_results_summary/pdf/ebr20120510_2.pdf [Last accessed 12 March 2015]
- Dainippon Sumitomo Pharma. Supplementary Financial Data for the Third Quarter of the Year Ending March 31, 2013. 2013. Available from: http://www.ds-pharma.com/ir/library/financial_results_summary/pdf/ebr20130131_2.pdf [Last accessed 12 March 2015]
- Jenkins Y, Sun TQ, Li Y, et al. Global metabolite profiling of mice with high-fat diet-induced obesity chronically treated with AMPK activators R118 or metformin reveals tissue-selective alterations in metabolic pathways. BMC Res Notes 2014;7:674
- Murray O; Robinson. Parkinson’s Disease Gene Networks. 2010. Available from: http://media.virbcdn.com/files/6d/78117666ecd66df9-PDGeneNetworksMolquant.pdf [Last accessed 13 March 2015]
- PR Newswire. Rigel Announces Publication Of R118 AMPK Activator Research. 2014. Available from: http://www.thestreet.com/story/12519501/1/rigel-announces-publication-of-r118-ampk-activator-research.html [Last accessed 23 March 2015]
- PR Newswire. R348 Did Not Meet Endpoints in Phase 2 Dry Eye Study. 2014. Available from: http://www.thestreet.com/story/12843148/1/r348-did-not-meet-endpoints-in-phase-2-dry-eye-study.html [Last accessed 23 March 2015]
- Wikipedia. Prostaglandin. 2014. Available from: http://en.wikipedia.org/wiki/Prostaglandin#cite_note-isbn0-87893-617-3-1 [Last accessed 6 March 2015]
- ONO PHARMACEUTICAL. Announcement on Co-development and Co-marketing Agreement for Carpal-tunnel Syndrome, an Additional Indication of Limaprost, an Oral Prostaglandin E1 Analogue. 2011. Available from: http://www.ono.co.jp/eng/news/pdf/sm_cn110601.pdf [Last accessed 23 March 2015]
- Sumitomo Dainippon Pharma. Discontinuation of Development for the Additional Indication of Carpal-Tunnel Syndrome, for Limaprost, an Oral Prostaglandin E1 Analogue. 2013. Available from: http://www.ds-pharma.com/news/2013/20130125.html [Last accessed 6 March 2015]
- ONO PHARMACEUTICAL. Discontinuation of Development for an Additional Indication of Cervical Spondylosis of Limaprost Alfadex, an Oral Prostaglandin E1 Derivative Preparation. 2008. Available from: http://www.ds-pharma.com/pdf_view.php?id=145 [Last accessed 23 March 2015]
- Clinicaltrials.gov. The Study About the Efficacy of Oral Limaprost After Surgery for Cervical Myelopathy. 2014. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02125981?term=limaprost+alfadex&rank=1 [Last accessed 6 March 2015]
- Taisho Pharmaceuticals. Taking Resolute Steps in a Shifting Market Annual Report 2008. 2008. Available from: http://www.taisho-holdings.co.jp/ir/library/pdf/annual/08_all.pdf [Last accessed 6 March 2015]
- Brass EP, Anthony R, Cobb FR, et al. The novel phosphodiesterase inhibitor NM-702 improves claudication-limited exercise performance in patients with peripheral arterial disease. J Am Coll Cardiol 2006;48(12):2539–45
- Ishiwata N, Noguchi K, Kawanishi M, et al. NT-702 (parogrelil hydrochloride, NM-702), a novel and potent phosphodiesterase inhibitor, improves reduced walkingdistance and lowered hindlimb plantar surface temperature in a rat experimental intermittent claudication model. Life Sci 2007;81(21):970–8
- Clinicaltrials.gov. Efficacy and Safety of NM-702 Tablets for the Treatment of Intermittent Claudication. 2006. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00102050?term=nm-702&rank=1 [Last accessed 6 March 2015]
- Taisho Pharmaceutical Co Ltd. Second Quarter of FY2014. 2015. Available from: http://www.taisho-holdings.co.jp/en/ir/library/pdf/presentation/14_1031_01-e.pdf [Last accessed 3 April 2015]
- Taisho Pharmaceutical Co Ltd. Discontinuation of Development of NT-702 for a Part of Indications. 2009. Available from: http://www.taisho.co.jp/en/company/release/2009/2009082601-e.pdf [Last accessed 3 April 2015]
- Kyowa Hakko Kirin Co., Ltd. Kyowa Hakko Kirin Announces the Development Status of Bardoxolone Methyl (RTA 402) in Patients with Chronic Kidney Disease and Type 2 Diabetes in Japan. 2013. Available from: http://kyowa-kirin.com/news_releases/2013/e20131111_01.html [Last accessed 10 March 2015]
- Kyowa Hakko Kirin Co., Ltd. Kyowa Hakko Kirin and Reata Pharmaceuticals entered into a licensing agreement on bardoxolone methyl in Japan and other selected Asian markets. 2010. Available from: http://kyowa-kirin.com/news_releases/2010/e20100108_01.html [Last accessed 10 March 2015]
- Kyowa Hakko Kirin Co., Ltd. Future development direction for bardoxolone methyl (RTA 402). 2014. Available from: http://kyowa-kirin.com/news_releases/2014/e20140702_01.html [Last accessed 10 March 2015]
- Hong DS, Kurzrock R, Supko JG, et al.A Phase I. First-in-Human Trial of Bardoxolone Methyl in Patients with Advanced Solid Tumors and Lymphomas. Clin Cancer Res 2012;18(12):3396–406
- Inazu A, Brown ML, Hesler CB, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med 1990;323(18):1234–8
- Roche. Roche provides update on Phase III study of dalcetrapib. 2012. Available from: http://www.roche.com/media/store/releases/med-cor-2012-05-07.htm [Last accessed 10 March 2015]
- Schwartz GG, Olsson AG, Abt M, et al. dal-OUTCOMES Investigators Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367(22):2089–99
- Japan Tobacco. JT’s Consolidated Financial Results for the 12 months ended March 31, 2012. 2012. Available from: http://www.jti.com/files/5413/3542/0077/FINAL_JTs_Consolidated_Financial_Results_for_the_12_months_ended_March_31_2012.pdf [Last accessed 10 March 2015]
- Tokyo. JT’s consolidated financial results for FY2013 first quarter. 2013. Available from: http://www.jt.com/media/press_releases/2013/pdf/20130730_02.pdf [Last accessed 23 March 2015]
- Lippiello PM, Beaver JS, Gatto GJ, et al. TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity. CNS Neurosci Ther 2008;14(4):266–77
- Targacept, Inc. Targacept announces plans to develop enantiomer of mecamylamine as augmentation therapy for depression. 2007. Available from: http://www.medicalnewstoday.com/releases/86693.php [Last accessed 10 May 2015]
- Fedorov N, Moore L, Gatto G, et al. Differential effects of TC-5214[S-(+)-mecamylamine] and TC-5213[R-(-)-mecamylamine] at low and high sensitivity human alpha4beta2 nicotinic receptors and in animal models of depression and anxiety. TC 2007;100:150
- AstraZeneca. A Study to assess the efficacy and safety of TC-5214 as an adjunct therapy in patients with major depressive disorder. 2010. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01197508?term=NCT01197508&rank=1 [Last accessed 23 March 2015]
- AstraZeneca. A study to assess the efficacy and safety of TC-5214 as an adjunct therapy in patients with major depressive disorder. 2010. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01180400?term=NCT01180400&rank=1 [Last accessed 23 March 2015]
- AstraZeneca. A study to assess the long- term safety of TC-5214 as an adjunct therapy in patients with major depressive disorder. 2010. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01152554?term=NCT01152554&rank=1 [Last accessed 23 March 2015]
- AstraZeneca. A study to assess the efficacy and safety of TC-5214 as an adjunct therapy in patients with major depressive disorder. 2010. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01153347?term=NCT01153347&rank=1 [Last accessed 23 March 2015]
- AstraZeneca. A study to assess the efficacy and safety of TC-5214 as an adjunct therapy in patients with major depressive disorder (MDD). 2010. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01157078?term=NCT01157078&rank=1 [Last accessed 23 March 2015]
- Press Release. AstraZeneca and targacept announce remaining TC-5214 phase 3 efficacy studies do not meet primary endpoint, regulatory filing will not be pursued. 2012. Available from: http://www.targacept.com/newsroom/index.cfm?nid=AstraZeneca%20and%20Targacept%20Announce%20Remaining%20TC%2D5214%20Phase%203%20Efficacy%20Studies%20Do%20Not%20Meet%20Primary%20Endpoint%2C%20Regulatory%20Filing%20Will%20Not%20Be%20Pursued&newsyear=2012 [Last accessed 23 March 2015]
- Press Release. Targacept announces plans to develop TC-5214 in overactive bladder. 2012. Available from: http://www.targacept.com/newsroom/index.cfm?nid=Targacept%20Announces%20Plans%20to%20Develop%20TC%2D5214%20in%20Overactive%20Bladder&newsyear=2012 [Last accessed 23 March 2015]
- Press Release. Targacept announces initiation of phase 2b study of TC-5214 in overactive bladder. 2013. Available from: http://www.targacept.com/newsroom/index.cfm?nid=Targacept%20Announces%20Initiation%20of%20Phase%202b%20Study%20of%20TC%2D5214%20in%20Overactive%20Bladder&newsyear=2013 [Last accessed 23 March 2015]
- Press Release. Targacept to discontinue TC-5214 overactive bladder program. 2014. Available from: http://www.targacept.com/newsroom/index.cfm?nid=Targacept%20to%20Discontinue%20TC%2D5214%20Overactive%20Bladder%20Program&newsyear=2014 [Last accessed 23 March 2015]
- Press Release. Targacept reports second quarter 2009 financial results. 2009. Available from: http://www.targacept.com/newsroom/index.cfm?nid=Targacept%20Reports%20Second%20Quarter%202009%20Financial%20Results&newsyear=2009 [Last accessed 23 March 2015]
- Teijin. Consolidated financial statements summary. 2009. Available from: http://www.teijin.com/ir/library/consolidated_results/pdf/cr_091102.pdf [Last accessed 23 March 2015]
- John Carroll. BMS offers up to $385M to develop atrial fibrillation drug. 2009. Available from: http://www.fiercebiotech.com/story/bms-offers-385m-develop-atrial-fibrillation-drug/2009-03-05 [Last accessed 11 March 2015]
- Nissan Chemical Industries, Ltd. Annual report 2010 11. Available from: http://www.nissanchem.co.jp/ir_info/archive/ar/ar2010.pdf [Last accessed 11 March 2015]
- John Carroll. Isis dumps rheumatoid arthritis effort after antisense drug flunks PhII. 2013. Available from: http://www.fiercebiotech.com/story/isis-dumps-rheumatoid-arthritis-effort-after-antisense-drug-flunks-phii/2013-08-05 [Last accessed 12 March 2015]
- Roche. Media release. 2012. Available from: http://www.roche.com/media/store/releases/med-cor-2012-09-05.htm [Last accessed 13 March 2015]
- ClinicalTrials. A phase II study of the safety and efficacy of MPSK3169A in patients with coronary heart disease or high risk of coronary heart disease. 2012. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01609140?term=NCT01609140&rank=1 [Last accessed 13 March 2015]
- Roche. Pipeline summary. 2014. Available from: http://www.roche.com/irp2q14e-annex.pdf [Last accessed 13 March 2015]
- Zhang XS, Xiang BR. Discontinued drugs in 2007: cardiovascular drugs. Expert Opin Investig Drugs 2008;17(12):1817–28
- Zhang XS, Xiang BR. Discontinued drugs in 2008: cardiovascular drugs. Expert Opin Investig Drugs 2009;18(7):875–85
- Zhao HP, Zhang XS, Xiang BR. Discontinued drugs in 2010: cardiovascular drugs. Expert Opin Investig Drugs 2010;20(10):1311–25
- Zhao HP, Jiang HM, Xiang BR. Discontinued drugs in 2012: cardiovascular drugs. Expert Opin Investig Drugs 2013;22(11):1437–51
- Zhao HP, Jiang HM, Xiang BR. Discontinued drugs in 2011: cardiovascular drugs. Expert Opin Investig Drugs 2012;21(10):1449–62
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 2004;3(8):711–16
- AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365(24):2255–67
- Tonkin AM, Chen L. Effects of combination lipid therapy in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010;122(8):850–2
- Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol 2012;59(25):2354–5
- Sanofi. Sanofi and regeneron present detailed positive results from four pivotal alirocumab trials at ESC Congress. 2014. Available from: http://en.sanofi.com/Images/37129_20140831_AlirocumabESCDataRelease_en.pdf [Last accessed 28 March 2015]
- AMGEN. Amgen announces positive top-line results from phase 3 TESLA trial of evolocumab (AMG 145) in patients with homozygous familial hypercholesterolemia. 2014. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01609140?term=NCT01609140&rank=1 [Last accessed 28 March 2015]
- Randi Hernandez. Amgen Sues sanofi and regeneron over patent for mAb targeting PCSK9. 2014. Available from: http://www.biopharminternational.com/amgen-sues-sanofi-and-regeneron-over-patent-mab-targeting-pcsk9 [Last accessed 28 March 2015]
- Sanofi. Sanofi and Regeneron Announce Praluent™ (alirocumab) Biologics License Application has Been Accepted for Priority Review by US FDA. 2015. Available from: http://en.sanofi.com/Images/38242_20150226_alirocumabbla_en.pdf [Last accessed 28 March 2015]
- AMGEN. Amgen Submits Application For Investigational LDL Cholesterol-Lowering Medication Repatha™ (evolocumab) In Japan. 2015. Available from: http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2015&releaseID=2027353 [Last accessed 28 March 2015]

