Abstract
Importance of the field: Hepatic encephalopathy (HE) is a major complication encountered in nearly half of the patients with liver cirrhosis.
Areas covered in this review: A review of the safety and efficacy of current therapies for HE that seek to pre-empt ammonia production and/or to increase its elimination, reducing inflammation, blocking benzodiazepine-like compound production, and supporting systemic hemodynamics.
What the reader will gain: Insight into some recent advances in the management of HE that could modify our therapeutic approach to end-stage liver disease. Cirrhotic individuals during an overt HE episode require careful management, focusing on precipitant factors as well as metabolic and hemodynamic derangements.
Take home message: Intestinal ammoniagenesis requires flora modification by antibiotics, prebiotics and probiotics; glutaminase inhibition as well as antibiotics to pre-empt systemic inflammation. Hemodynamic/fluid support is essential. Nutritional support is crucial and hypoproteinemic diets should be avoided. Blocking benzodiazepine-like compounds by the use of flumazenil could be useful in patients with severe, benzodiazepine-induced HE. Long-term rifaximin is well tolerated, does not promote resistance and could decrease overt HE bouts in patients with previous episodes of overt HE. Lactulose is better than no treatment in improving quality of life in patients with minimal HE; it also acts as secondary prophylaxis following overt HE.
1. Introduction
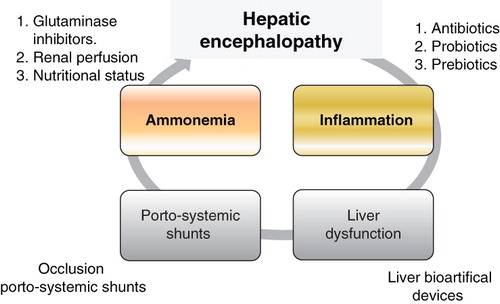
Hepatic encephalopathy (HE) is one of the major complications of liver cirrhosis. It has a considerable socioeconomic impact as it reduces the individual's quality of life and is considered to be a symptom of advanced liver disease, with a clinical indication for liver transplantation. HE can be defined, after exclusion of other brain diseases, as a complex neuropsychiatric syndrome that occurs in patients with liver dysfunction. HE is classified into three types Citation[1]: type A, associated with acute liver failure; type B, associated with the existence of porto-systemic communications without intrinsic liver disease; and type C, associated with cirrhosis, which, depending on the manner of presentation, is classified into episodic HE (related to precipitating factors) or spontaneous (persistent HE). This latter class is further subdivided into mild (grade I HE), severe (HE II – IV) or treatment-dependent (early development after treatment cessation), and minimal HE as the first manifestation of HE. The differential diagnosis in clinical practice is often easier when a cirrhotic patient develops behavioral disorder, temporal–spatial disorientation or coma. However, it is sometimes difficult to distinguish minimal HE from normal, or grade 1 HE. The two key factors for the development of HE are hepatic dysfunction and porto-systemic communication (). The presence of large porto-systemic communications, either spontaneous or surgical, may influence the development of HE events, despite preserved liver function. Severe liver dysfunction in the context of terminal illness, advanced cancer, or acute deterioration of chronic liver disease is characterized by the appearance of HE in the absence of other precipitating factors, or large porto-systemic communications. Precipitating factors of HE can be classified according to the pathogenic events that determine its onset: i) intestinal ammoniagenesis: constipation, excessive protein intake, increased glutaminase activity that occurs with increased intestinal production of ammonia; ii) inflammatory systemic response: infections such as spontaneous bacterial peritonitis, urinary tract infection, pneumonia, or in soft tissue as well as complex surgical procedures or coexistence of inflammation or neoplasia, would lead to a state of systemic inflammatory response; iii) increased GABAergic tone: the use of psychoactive drugs, mainly benzodiazepines increase GABA tone; and iv) renal ammoniagenesis: gastrointestinal bleeding via esophago-gastric varices leads to hemodynamic disturbances which appear to be responsible for the onset of HE, similar to that associated with renal failure, hyponatremia, hypokalemia, dehydration, or use of nephrotoxic agents.
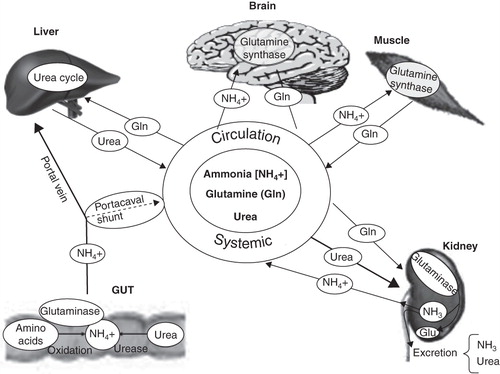
Figure 1. Interorgan ammonia trafficking. Ammonia derived from gut and kidneys, mainly due to glutaminase activity, must be detoxified in muscles because of possible liver dysfunction. Systemic hyperammonemia promotes brain uptake and induces low-grade cerebral edema, as well as alterations in neurotransmission.
This article reviews the current treatment of HE. The therapeutic options focus on achieving a lower production of ammonia, increasing its elimination, improving the systemic hemodynamic status, and preventing the development of systemic inflammatory response. The occlusion of large porto-systemic shunts has been found useful in patients with preserved liver function. Finally, as the development of an episode of HE is accompanied by a low 1-year survival rate (estimated between 42 and 58%), liver transplantation should be the definitive treatment for this major complication of liver cirrhosis.
2. Pathophysiology of hepatic encephalopathy
2.1 Hyperammonemia
Ammonia plays a major role in the pathogenesis of HE. Systemic hyperammonemia has been well documented in patients with cirrhosis, and increased ammonia in brain has been linked to astrocyte edema and impairment in the neurotransmission leading to HE. For many years, ammonemia has been considered as being derived from urea breakdown by intestinal bacteria. As such, the majority of treatments were targeted against colon-associated bacteria-derived ammonia Citation[2]. However, the hyperammonemia following portacaval shunt in rats has been found to be similar in germ-free as in non-germ-free animals Citation[3,4]. This finding provides support for the proposal that hyperammonemia and encephalopathy could develop without the participation of bacteria Citation[5]. For example, the highest degree of hyperammonemia in cirrhotic patients has been found in portal-drained viscera and derived, mainly, from glutamine deamination Citation[6]. Indeed, the area under the curve (AUC) of oral glutamine challenge correlates with liver dysfunction and minimal HE Citation[7]. Phosphate-activated glutaminase (PAG) catalyzes the hydrolysis of glutamine (Gln) to yield glutamate (Glu), energy, nucleotide synthesis and ammonia Citation[8]. Duodenal PAG activity has been found to be nearly 4-fold higher in cirrhotic patients than in healthy controls Citation[9]. Moreover, PAG activity was closely related to minimal HE and correlated with choline/creatine and Gln/Glu (Glx)/creatine intracerebral ratio Citation[10]. As the main source of ammonia in cirrhotic patients results from portal-drained viscera, systemic hyperammonemia appears to be a consequence of glutamine deamidation by PAG Citation[11]. Further, in porto-caval shunted rats (a model of chronic hyperammonemia) intestinal activity of PAG has been found to be increased relative to sham-operated rats Citation[12].
Data supporting a genetic factor influencing the development of overt HE include: i) < 40% of cirrhotic patients with minimal HE do not develop overt HE despite follow-up > 7 years; ii) cirrhotic patients showing the same degree of liver dysfunction and suffering from the same precipitant factor (i.e., variceal bleeding) may, or may not, develop overt HE; and iii) Taylor et al. described at least two different polymorphisms in the promoter region of K-glutaminase gene that could influence protein activity in either direction (an increase or a decrease) in PAG activity Citation[13]. The human glutaminase gene (OMIM: 138280) is located on chromosome 2 (2q32-q34). The full-length gene includes 84,675 base-pairs (bp) and the glutaminase mRNA has 4784 bp. It comprises 18 exons and 17 introns (GenBank NM_014905) Citation[14]. Recently, a functional microsatellite in the promoter region of the glutaminase gene has been linked to the risk of overt HE in cirrhotic patients and, in the longer of the allelic forms, is associated with increased enzyme activity. This genetic marker could help in identifying patients at risk of overt HE and, hence, they could be carefully monitored and/or receive intensive treatment. This microsatellite would influence cirrhosis management, and could be useful as a pharmacogenetic indicator of therapeutic options in HE Citation[15].
In patients suffering from HE as a complication of diuretic treatment, or after variceal bleeding, the kidney seems to play a major role in the production of hyperammonemia. In this situation saline infusions could improve the status of the HE Citation[16].
2.2 Inflammation
Since Shawcross et al. reported the impact of systemic inflammatory response on ammonia-induced brain dysfunction in cirrhotic patients Citation[17], the role of inflammation in the pathogenesis of HE has been addressed in several ways. Improving inflammation using antibiotics or non-steroidal anti-inflammatory drugs (NSAID) prevents brain dysfunction. In cirrhotic patients with hyperammonemia following the ingestion of an amino-acid solution mimicking digestive bleeding, clinical manifestations of systemic inflammation and HE were observed. Together with clinical signs, inflammation was assessed in terms of nitric oxide, interleukin-6, interleukin-1β and tumor necrosis factor (TNF) levels. When the solution was given to patients with systemic infection, hyperammonemia was associated with signs and symptoms of HE. However, when infection was resolved with antibiotics and systemic inflammation disappeared (demonstrated by a normalization of cytokine levels) clinical HE did not develop despite the same level of hyperammonemia following the amino acid intake Citation[17]. Inflammation in cirrhotic patients without infections seems to derive from bacterial translocation in the intestine. Probiotics (mainly saprophytic bacteria) and prebiotics (non-absorbable disaccharides) have been found useful in the management of minimal HE. Together with the ability to decrease ammonia production in the large intestine, probiotics could avoid bacterial translocation and systemic inflammation, thus decreasing brain damage induced by ammonia Citation[18]. Further, in portacaval shunted rats, treatment with ibuprofen for 18 days restored learning ability Citation[19].
3. Therapeutic options in hepatic encephalopathy
In the management of overt HE it is crucial to: i) identify and correct for the precipitating factors; ii) rule out other causes of brain impairment in cirrhosis (such as intracranial hematomas, drug intoxication, sepsis, thyroid dysfunction, hypoglycemia, hyperglycemia, encephalitis, uremia, hypoxemia, hypercapnia); iii) evaluate gastrointestinal bleeding, metabolic abnormalities or infections; iv) eliminate sedatives as a cause; v) administer intravenous fluid; and vi) correct for electrolyte abnormalities (). Specific measures include: reduction of intestinal ammonia production, preservation of nutritional status, and reduction in the flow in portal-systemic shunts ().
3.1 Reduction of ammonia production
HE occurs as a result of the coexistence of two pathologies: hyperammonemia and inflammation. Control of HE signs and symptoms is achieved by eliminating these conditions Citation[20]. Bacterial translocation from the gut is the major cause of systemic inflammatory response in cirrhotic patients and the use of antibiotics, prebiotics and probiotics is essential for the management of cirrhotic patients with HE.
3.2 Antibiotics
Antibiotics, especially neomycin, paromomycin, metronidazole, and rifaximin, have been demonstrated to be useful in the treatment of HE. Antibiotics have been found to be superior to non-absorbable disaccharides for the improvement of HE, decreasing blood ammonia and improving psychometric test scores. The mechanisms of action are multiple. Antibiotics remove ammonia-producing bacterial flora and lower bacterial translocation and systemic inflammation. In some cases, such as neomycin, the antibiotic has been shown to inhibit, at least partially, the intestinal glutaminase activity Citation[12]. The main limitations of prolonged antibiotic therapy are the adverse events (frequently seen in aminoglycosides) and induction of resistance (e.g., in the case of metronidazole). Non-absorbed oral antibiotic therapy has been widely used for the treatment of HE.
Neomycin was the first antibiotic to be used. This has affects the gut colon bacteria and also reduces the production of ammonia from glutamine in the gut. However, neomycin has important, non-reversible inconveniences, i.e., ototoxicity and nephrotoxicity, in just under 10% of the patients.
Rifaximin is a semi-synthetic, non-systemic antibiotic derivative of rifamycin with a wide spectrum of antimicrobial activity and low gastrointestinal absorption (0.5%) and, as such, has almost no adverse effects and no resistance develops Citation[21]. Rifaximin inhibits RNA synthesis and shows antibacterial activity against Gram-positive and Gram-negative bacteria, aerobes and anaerobes. Rifaximin reduces stool concentration of bacteria in the first week of treatment. However, the effect is short-lasting as bacterial populations recover within 1 – 2 weeks following the conclusion of treatment. Resistances are not detectable after 3 months of therapy Citation[22,23]. The lack of these resistances encourages the cyclic or long-term use of rifaximin in the treatment of HE. When rifaximin and non-absorbable disaccharides were compared in different studies, rifaximin was demonstrated to be better in improving the degree of HE as well as its signs and symptoms, with almost no side effects Citation[24]. In a study by Mas et al., which compared rifaximin versus lactitol in the treatment of cirrhotic patients with grade I – III acute or recurrent HE, rifaximin significantly improved ammonia levels and porto-systemic encephalopathy index (PSE) index in comparison with lactitol. However, the percentage of patients with complete HE resolution was similar in both treatment groups Citation[25]. Other studies have compared rifaximin with other antibiotics and have observed that rifaximin caused a more rapid and significant decrease in ammonia levels than other antibiotics, and in a safer manner Citation[26].
Hence, in patients with HE, rifaximin is as good or better than other antibiotics and non-absorbable disaccharides, and achieves early clinical improvement with better tolerance by the patient Citation[27]. Further, rifaximin has been found to be superior to placebo as secondary prophylaxis. In a prospective, randomized, double-blind trial, rifaximin was compared to placebo in a cohort of 299 cirrhotic patients with at least two episodes of HE grade II or higher in the previous 6 months. Patients received 1100 mg of rifaximin daily or placebo for 6 months. Prolonged use of rifaximin resulted in a decrease in episodes of encephalopathy during the follow-up (22 vs 46%; p = 0.0001). Rifaximin improved critical flicker frequency and blood ammonia level, but not the patient's quality of life Citation[28]. Rifaximin is cost effective; its use is associated with reduced hospitalizations, shorter hospital stays and cost savings Citation[29]. Despite a daily dose of rifaximin being more expensive than lactitol, patients treated with this drug have less hospitalizations and a shorter stay in hospital and, hence, a full course of rifaximin treatment would be cheaper than lactitol Citation[30].
3.3 Probiotics
Probiotics are living, non-pathogenic micro-organisms, which, as food ingredients, benefit the host's health. Probiotics can be lactic bacteria (Lactobacillus or Bifidobacterium) or yeasts (Sacharomyces), which, in the small intestine, can promote the fermentation of non-adsorbed sugars such as glucose, fructose, sucrose and lactose as well as prebiotic fibers such as fructose-derived oligo- and polysaccharides (inulin). Probiotics pre-empt fermentation by other bacteria, encourage the elimination of selective micro-flora, reduce the production of ammonia and limit bacterial translocation. These effects decrease total ammonia in portal blood, decrease inflammation and oxidative stress in the hepatocytes, and result in a decrease in uptake of microbial toxins and toxic metabolites. Probiotics have been administered in different forms and combinations, e.g., symbiotic preparation with four freeze-dried, non-urease-producing bacteria such as Pediacoccus pentoseceus 5-33:3, Leuconostoc mesenteroides 32-77:1, Lactobacillus paracasei subspecies paracasei 19 and Lactobacillus plantarum 2592, each at a dose of 1010 colony forming units (CFU) per sachet. An alternative administration is VSL#3®, which consists of sachets containing 900 billion viable lyophilized bacteria comprising four strains of Lactobacillus (L. paracasei, L. plantarum, L. acidophilus and L. delbrueckii subsp. bulgaricus), three strains of Bifidobacterium (B. longum, B. breve and B. infantis) and one strain of Streptococcus thermophilus. Recently, Lactobacillus GG has been selected for a long-term, double-blind, trial in the USA in patients with minimal HE.
Probiotics have been found to be beneficial in the management of minimal HE. In a cohort of 60 cirrhotic individuals randomized to receive bifidobacterium + fructo-oligosaccharides (FOS) or placebo over a period of 3 months, probiotics were able to decrease fasting ammonia levels resulting in significant improvements in psychometric test performances (including: Trail Making Test-A and B, symbol digit modalities test, block design test) relative to placebo Citation[19]. Further, in a cohort of 105 patients with minimal HE randomized to receive probiotics, or lactulose, or both, a similar effect in the three treatment arms was found. The three treatments resulted in clearance of minimal HE in just over half of the cases Citation[31]. Loguercio et al. demonstrated that Enterococcus faecium SF68 was superior to lactulose in improving ammonia levels, in number connection tests, and flash-evoked visual potential in 40 cirrhotic individuals receiving 1 g/kg bodyweight/day proteins Citation[32]. Further, Malaguarnera et al. reported a double-blind comparison of FOS-containing bifidobacterium versus lactulose. In 125 patients with minimal HE treated with probiotics or lactulose for 60 days, bifidubacterium + FOS significantly improved hyperammonemia and psychometric test results (trail-making-test B, block design test and digit symbol test, BDT, and DST) compared to lactulose Citation[33]. Moreover, in a study comparing probiotic yoghurt versus no treatment, excellent treatment adherence, no adverse effects, and beneficial effects on the cytokine profile, blood ammonia levels and psychometric test scores were noted Citation[34]. Indeed, Liu et al. compared the effect of probiotics (oral supplementation with four freeze-dried, non-urease-producing bacteria) together with 10 g of fermentable fiber (beta glucan 2.5 g; inulin 2.5 g; pectin 2.5 g; resistant starch 2.5 g) versus fiber alone or placebo. In 58 cirrhotic individuals with minimal HE, 4 weeks of therapy with symbiotics or fiber alone was associated with minimal HE disappearance in 50% of study subjects, together with decreased ammonia and endotoxin levels, improvement in liver function and acid pH < 5.5 in feces, compared to placebo Citation[35]. Thus, in the management of minimal HE, probiotics (mainly Lactobacillus) have been found to be superior to placebo, and equal or superior to lactulose. No significant improvement was observed when using the probiotic and the prebiotic together.
3.4 Prebiotics: non-absorbable disaccharides (lactulose and lactitol)
Lactulose or lactitol continue to be the main therapeutic options in the management of HE. Non-absorbable disaccharides (NADs) have been used in the treatment of HE because the laxative effect resolves constipation, promotes acidic stools, and decreases ammonia absorption by the gut Citation[36]. Studies analyzing the effects of NADs on HE are scarce, not well designed or have included a low number of cases. Meta-analyses comparing lactulose or lactitol with antibiotics have shown contradictory results. For example, Jiang et al. found a similar efficacy for antibiotics and lactulose/lactitol Citation[37]. However, Als-Nielsen et al. Citation[38] compared non-absorbable disaccharides with placebo or no intervention and found significant benefit [relative risk (RR) 0.62; 95% confidence interval (CI) 0.46 – 0.84]. The beneficial effects disappeared when only the high-quality studies were included (RR 0.92; 95% CI 0.42 – 2.04), and the results were similar to placebo. Therefore, an effectiveness of non-absorbable disaccharides in HE is observed only in low-quality studies conducted in patients with minimal EH that had not been properly defined and without follow-up results, i.e., reflecting a bias from poor quality methodology. The analyses showed that antibiotics are superior to non-absorbable disaccharides in improving HE, in decreasing blood ammonia and in improving psychometric test scores, together with a higher risk of non-improvement (RR 1.24; 95% CI 1.02 – 1.50). No differences were found with respect to mortality and safety.
The use of lactulose/lactitol enemas in everyday clinical practice is based on a single three-arm trial comparing enemas of lactitol, lactulose and water. The trial was stopped after an interim analysis because the results already showed a significant benefit (p = 0.004) of lactitol enemas compared with water enemas. To avoid false positives, the significance needed to be ≤ 0.001 and, hence, the interruption of the study is questionable, especially as no differences were found between lactulose and water enemas.
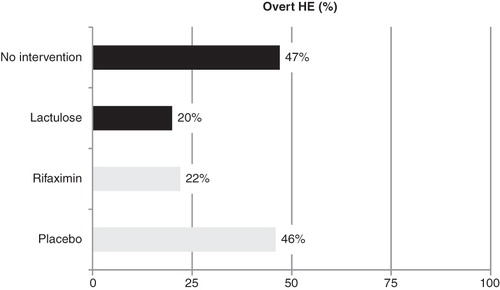
Recently, three studies assessing the efficacy of lactulose in the management of patients with HE have been reported. In a cohort of patients with minimal HE, lactulose treatment was compared to non-intervention. After 3 months of therapy, quality-of-life improved in patients receiving lactulose compared to untreated cirrhotic patients; this improvement correlated with positive changes in psychometric test scores Citation[39]. Further, in a non-blinded study, lactulose (30 – 60 ml/day) was compared with placebo in a cohort of patients with previous episodes of overt HE. The rate of onset of new episodes of HE in the follow-up was lower in patients receiving lactulose (12/61; 20%) compared with those receiving placebo (30/64; 47%; p < 0.001). Re-admission rates and mortality were similar in both groups Citation[40]. Interestingly, infection factors (i.e., spontaneous bacterial peritonitis or pneumonia) associated with overt HE during the follow-up were more often seen in the non-intervention group than in patients receiving lactulose. Finally, the predictors of non-response to lactulose were the degree of hyperammonemia (the higher ammonemia the poorer the response) and hyponatremia. These findings reaffirm the poor outcomes of the use of lactulose in patients with encephalopathy grade III – IV Citation[41]. Despite the simple design of these studies with a ‘hard’ primary end-point and including a large number of patients, the lack of a comparator (blinded arm) implies that caution needs to be exercised in interpreting these data ().
3.5 Nutritional status
A feed-forward loop applies in cirrhotic patients. Decreasing liver function and increasing porto-systemic shunting increases ammonia concentration. In turn, the muscles remove this ammonia via glutamine synthase and export glutamine, which leads to further ammonia production. Indeed, ammonia causes elevated skeletal muscle myostatin together with reduced myoblast proliferation and differentiation, and a decrease in the detoxification activity of muscles Citation[42]. Protein restriction has been another classic issue in the treatment of HE, the effectiveness of which has been widely questioned. Further, the most important pathway for ammonia elimination that does not require energy consumption is the urea cycle. In cirrhotic patients, most of the ammonia is removed from the muscle. Córdoba et al. Citation[43], found that protein restriction did not improve the clinical evolution of acute HE, and the restriction was also associated with alterations in nitrogen metabolism. The cirrhotic patients suffering from overt HE (n = 30) were randomized to receive a low-protein diet with progressive increments, or a normal protein diet, for 14 days together with standard measures to treat HE. Protein metabolism (synthesis and catabolism) was studied with the 15N-glycine infusion method. The low-protein diet group showed higher protein catabolism but the same rate of overt HE. The use of branched-chain amino acid (BCAA) preparations exerts a collateral beneficial effect on encephalopathy, probably derived from the improved nutritional status rather than the false modification of neurotransmitter balance Citation[44]. l-ornithine-l-aspartate is a complex amino acid that stimulates both the urea cycle and muscle protein synthesis. In two randomized, double-blind studies for the treatment of HE type C, l-ornithine-l-aspartate was superior to placebo in improving mental status, decreasing hyperammonemia, and improving psychometric test scores when administered orally Citation[45] or intravenously Citation[46]. A recent meta-analysis demonstrated the value of l-ornithine-l-aspartate in the treatment of patients with mild HE (grade I – II); no improvement was observed in patients with severe encephalopathy (grades III – IV) or in patients with minimal HE Citation[47]. The administration of intravenous l-ornithine-l-aspartate is more effective but less safe than the oral route, probably because, in oral administration, a part of the aspartate is transaminated in the intestinal mucosa. l-ornithine-l-aspartate decreases hyperammonemia by increasing glutamine synthesis. However, this amino acid is not a safe storage end-product of ammonia because it can be deaminated by glutaminase to produce hyperammonemia, again Citation[48].
3.6 Benzodiazepine-like antagonists
Among the therapeutic measures designed to modulate neurotransmission, flumazenil (a benzodiazepine receptor antagonist of GABA-A receptor complex) could be beneficial in approximately a third of patients, due to an improvement in short-term mental state Citation[49]; albeit survival or recovery rates are not affected. Flumazenil may be used as the drug of choice in patients with HE triggered by the use of exogenous benzodiazepines, and could be used as a second-line agent in patients with HE grade III – IV who may be non-responders to standard therapeutic measures Citation[50]. Indeed, flumazenil showed the ability to decrease GABAergic tone that had been increased by the synthesis of neurosteroids due to hyperammonemia, as well as the ability to block the effect of peripheral benzodiazepine-like substances synthesized in some parts of the gut.
3.7 Embolization of spontaneous porto-systemic shunts
In cirrhotic patients, the presence of large spontaneous porto-systemic shunts were more often seen in patients with persistent HE than in non-encephalopathic cirrhotic individuals Citation[51]. The embolization of these shunts facilitated the control of HE signs and symptoms Citation[52]. Thus, in patients with persistent HE, computer tomography-based angiography or magnetic resonance angiography imaging should be performed if the presence of large shunts is suspected. HE is a well-described possible complication of trans-hepatic porto-systemic shunt insertion in cirrhotic patients. In the case of non-response to standard care (including lactulose and/or antibiotics) the partial occlusion of the trans-jugular intra-hepatic porto-systemic shunt (TIPS) could improve the HE Citation[53]. Of note is that HE has been found less frequently after TIPS when using coated stents despite lower TIPS dysfunction rate (therefore, maintaining constant porto-caval flow across the shunt) Citation[54], probably related to the ability of these types of stent to pre-empt inflammatory response.
4. Conclusions
Treatment of HE remains a challenge in the twenty-first century. Ammonia and inflammation are two key features of HE. Antibiotics (mainly rifaximin), prebiotics (lactulose/lactitol), and probiotics (Lactobacillus and Bifidobacterium) modify intestinal flora, decrease ammonia production and pre-empt bacterial translocation. Rifaximin and lactulose are useful in the management of patients following overt HE. Nutritional status is crucial in the management of cirrhotic patients; hypoproteic diets should be avoided. Patients with persistent HE should be investigated for porto-systemic shunts. Occlusion of shunts has been proved useful in the management of HE. Liver transplantation is indicated in patients with bouts of overt HE.
5. Expert opinion
The evaluation of different treatments used in HE is complicated and is affected by various sources of biases, which are the main weaknesses in defining the first-line therapy for HE. Factors complicating the interpretation of meta-analysis and systematic reviews include: i) the prognosis of overt HE depends on the management of precipitant factors and underlying liver disease; ii) 40 – 70% of cases may improve with placebo, which, in turn, differs from no intervention; iii) drugs tested and shown to be effective are being used in different doses and over wide time-schedules; and iv) lack of accurate methods to quantify therapeutic effects.
Therefore, well-designed randomized, controlled trials (RCT) are required to define the first-line therapy for HE. Further, different end-points should be set when treating minimal or overt HE, and the outcomes should be analyzed separately. In patients with minimal HE, the main end-points should be quality of life, impact on traffic- or work-accidents, and rates of admission for bouts of overt HE, as well as safety and tolerance of the drug. In patients with clinically evident HE, the main end-point is resolution of neuropsychiatric disorders, reduction in re-admissions, and improvement in survival. RCTs are warranted to define the best therapeutic option in the management of HE.
Several points need to be emphasized. First, a recent RCT comparing rifaximin and placebo as secondary prophylaxis after overt HE bouts demonstrated the usefulness of rifaximin. Six months of therapy with 1100 mg per day of rifaximin decreased the bouts of overt HE during follow-up and improved ammonia levels, as well as the performance of critical flicker frequency, relative to placebo. However, rifaximin did not influence quality of life or survival rates. Second, lactulose has been the treatment of choice in the management of HE since the 1980s. However, well-designed RCTs comparing lactulose versus placebo or other therapeutic options are scarce. Recently, a trial comparing lactulose and no intervention demonstrated the usefulness of lactulose as secondary prophylaxis of overt HE in patients suffering bouts of HE. However, any active arm per se was found to be capable of improving HE, and even placebo has been found to be superior to no intervention; patients felt better managed and patient adherence to HE therapy improved. Thus, we need to be cautious in interpreting these results. Indeed, in a systematic review, lactulose was found superior to placebo in the overall analysis of the studies but, on selecting only high-quality published articles, lactulose was not found to be significantly different from placebo. Double-blind, placebo-controlled studies are warranted to define the role of lactulose in the management of HE and in secondary prophylaxis. Third, the deleterious effect of hyperproteic diets in intestinal ammonia production seems to be lower than the harmful effect of hypoproteic diet on nutritional status and in ammonia detoxification. Thus, in patients with liver cirrhosis, a protein intake of 1.2 g/kg/day is recommended to achieve a stable nitrogen balance. This intake maintains muscle mass, as most of the ammonium is metabolized in the muscle through the synthesis of glutamine by action of the glutamine synthetase enzyme. Contrary to some recommendations in textbooks and/or some expert opinions, hypoproteic diets should be proscribed in the management of cirrhotic patients with HE. Fourth, probiotics seem to be useful in the management of minimal HE but RCTs are needed to clarify this issue. There are several types of probiotics, the safety and adherence to which are not as yet sufficient to indicate probiotics as first-line therapy in minimal HE. Fifth, the occlusion of large spontaneous porto-systemic shunts seems to be an interesting approach to managing patients with ‘white’ HE. This method has been found to be useful in several cases of chronic HE unresponsive to standard care. Sixth, liver transplantation remains the most useful method of treating HE, and all patients suffering from a bout of overt HE should be placed on a waiting list for liver transplantation.
From a pharmacogenetic point of view, a genetic marker capable of predicting the risk of overt HE has been reported. Patients bearing the ‘long-long’ allele of a microsatellite in the 5' untranslated region (UTR) of the promoter region of the glutaminase gene showed a 3-fold higher risk of developing overt HE in follow-up. This genetic signal could be useful in predicting response to the different pharmacologic agents discussed in this current review.
Psychometric HE score (PHES) has been consensually proposed as the method for the diagnosis of minimal HE Citation[55]. Critical flicker frequency (CFF) seems to be a reliable method that is able to detect changes in mental status. CFF is not influenced by age or education level and is useful in monitoring changes, at least in early grades I and II of the disease Citation[56]. Visual or auditory evoked potential and CFF have been useful in monitoring the management of HE. However, concordance with other psychometric methods is low, due to the different areas of the brain explored by each of the methods. CFF seems able to correctly classify patients when CFF is < 38 Hz, and could detect changes brought-about by correct treatment Citation[57]. Further, CFF predicts the risk of overt HE Citation[58]. However, these methods need to be verified systematically for potential use in monitoring the effects of treatment.
HE emerges as a consequence of liver dysfunction and porto-systemic shunts in the presence of precipitant factors. Thus, the success of treatment of an episode of overt HE depends on the management of these three factors. HE is associated with poor prognosis and has become accepted as a clinical indication for liver transplantation. Separating liver dysfunction from neuropsychiatric abnormalities remains elusive. The management of patients with preserved liver function (‘the white encephalopathy’) mainly due to large porto-systemic shunts, is different from ‘yellow encephalopathy’ tightly linked to liver dysfunction. HE needs to be better characterized within the context of the different clinical scenarios. New drugs focusing on new concepts in the pathophysiology of HE need to be developed, e.g., ornithine–phenylacetate or other glutaminase inhibitors, or drugs able to pre-empt ammonia accumulation as glutamine. New strategies supported by stem cells to improve liver function should be applied to HE. Finally, genetic studies could help to demonstrate new elements implicated in the development of overt HE, but also to define more clearly the profile of responders to different drugs. This would enable the appropriate therapy to be administered based on the patient's prognosis, as defined by the genetic profile.
Article highlights.
Ammonia and inflammation are key points in the pathophysiology of hepatic encephalopathy (HE).
Nutritional support is crucial in the management of HE; hypoproteinemic diets should be avoided.
Probiotics seem to be useful in minimal HE but further randomized, placebo-controlled trials are warranted.
Long-term rifaximin or lactulose should be used as secondary prophylaxis of overt HE.
Occlusion of porto-systemic shunts has been proved useful in the management of chronic HE.
Genetic alterations in the promoter region of the glutaminase gene predict risk of overt HE in compensated cirrhotic patients.
Declaration of interest
The author states no conflict of interest and has received no payment in preparation of this manuscript.
Acknowledgements
This study was supported by grants form the Spanish Ministry of Health (Instituto de Salud Carlos III: PI040384 and PI070425). Editorial assistance was by PR Turner.
Notes
This box summarizes key points contained in the article.
Bibliography
- Ferenci P, Lockwood A, Mullen K, Hepatic encephalopathy- Definition, Nomenclature, Diagnosis, and Quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35(3):716-21
- Sherlock S. Chronic portal-systemic encephalopathy: update 1987. Gut 1987;28:1043-8
- Nance FC, Kline DG. Eck's fistula encephalopathy in germ-free dogs. Ann Surg 1971;174:856-61
- Warren KS, Newton WL. Portal and peripheral blood ammonia concentrations in germ-free and conventional guinea pigs. Am J Pysiol 1959;197:717-20
- Weber FJL, Veach GL. The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterology 1979;77:235-40
- Olde Damink SW, Jalan R, Redhead DN, Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 2002;36:1163-71
- Romero-Gomez M, Grande L, Camacho I. Prognostic value of altered oral glutamine challenge in patients with minimal hepatic encephalopathy. Hepatology 2004;39:939-43
- James LA, Lunn PG, Middleton S, Distribution of glutaminase and glutamine synthase activities in the human gastrointestinal tract. Clin Sci 1998;94:313-9
- Romero-Gomez M, Ramos-Guerrero R, Grande L, Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004;41:49-54
- Romero-Gomez M. Role of phosphate-activated glutaminase in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 2005;20:319-25
- Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 1995;15:133-59
- Hawkins RA, Jessy J, Mans AM, Neomycin reduces the intestinal production of ammonia from glutamine. Adv Exp Med Biol 1994;368:125-34
- Taylor L, Liu X, Newsome W, Isolation and characterization of the promoter region of the rat kidney-type glutaminase gene. Biochim Biophys Acta 2001;1518:132-6
- Elgadi KM, Meguid RA, Qian M, Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol Genomics 1999;1:51-62
- Del Campo JA, Jover M, Royo JL, A microsatellite in the promoter region of the GLS gene predicts the risk of hepatic encephalopathy: functional analysis using luciferase reporter. Hepatology 2009;50:320A
- Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond) 2004;106:467-74
- Shawcross DL, Davies NA, Williams R, Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 2004;40:247-54
- Malaguarnera M, Greco F, Barone G, Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci 2007;52:3259-65
- Cauli O, Rodrigo R, Piedrafita B, Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology 2007;46:514-9
- Wright G, Jalan R. Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: pandora's box? Hepatology 2007;46:291-4
- Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion 2006;73(Suppl 1):13-27
- Testa R, Eftimiadi C, Sukkar GS, A non-absorbable rifamycin for treatment of hepatic encephalopathy. Drugs Exp Clin Res 1985;11:387-92
- De Leo C, Eftimiadi C, Schito GC. Rapid disappearance from the intestinal tract of bacteria resistant to rifaximin. Drugs Exp Clin Res 1986;12:979-81
- Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin 1993;13:109-18
- Mas A, Rodés J, Sunyer L, Comparison of rifaximin and actitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol 2003;38:527-8
- Festi D, Vestito A, Mazzella G, Management of hepatic encephalopathy: focus on antibiotic therapy. Digestion 2006;73(Suppl 1):94-101
- Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacology 2008;28:1019-32
- Bass NM, Mullen KD, Sanyal A, Rifaximin Treatment in Hepatic Encephalopathy. N Eng J Med 2010;362:1071-81
- Leevy B, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Schi 2007;52:737-41
- Maclayton DO, Eaton-Maxwell Al. Rifaximin for treatment of hepatic encephalopathy. Ann Pharmacother 2009;43:77-84
- Sharma P, Sharma BC, Puri V, An open-label randomized controlled trial of lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol 2008;20:506-11
- Loguercio C, Abbiati R, Rinaldi M, Long-term effects of Enterococcus faecium SF68 versus lactulose in the treatment of patients with cirrhosis and grade 1-2 hepatic encephalopathy. J Hepatol 1995;23:39-46
- Malaguarnera M, Gargante MP, Malaguarnera G, Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol 2010;22(2):199-206
- Bajaj JS, Saeian K, Christensen KM, Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol 2008;103(7):1707-15
- Liu Q, Duan ZP, Ha DK, Symbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441-9
- Masini A, Efrati C, Merli M, Effect of lactitol on blood ammonia response to oral glutamine challenge in cirrhotic patients: evidence for an effect of non-absorbable disaccharides on small intestine ammonia generation. Am J Gastroenterol 1999;94:3323-7
- Jiang Q, Jiang XH, Zheng MH, Rifaximin versus non-absorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol 2008;20:1064-70
- Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ 2004;328:1046
- Prasad S, Dhiman RK, Duseja A, Lactulose improves cognitive function and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007;45:549-59
- Sharma BC, Sharma P, Agrawal A, Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology 2009;137:885-91
- Sharma P, Sharma BC, Sarin SK. Predictors of nonresponse to lactulose for minimal hepatic encephalopathy in patients with cirrhosis. Liver Int 2009;29:1365-71
- Dasarathy S, Yang Y, Muc S, Ammonia causes elevated skeletal muscle myostatin and reduced myoblast proliferation and differentiation. Hepatology 2009;50:22A
- Córdoba J, López-Hellín J, Planas M, Normal protein diet for episodic hepatic encephalopathy. J Hepatol 2004;41:38-43
- Les I, Planas M, Cardenas G, Effects of the proteins of the diet in patients with cirrhosis and prior episode of hepatic encephalopathy. A long-term randomized study. Hepatology 2009;50:24A
- Stauch S, Kircheis G, Adler G, Oral L-ornithine-L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol 1998;28:856-64
- Kircheis G, Nilius R, Held C, Therapeutic efficacy of L-ornitine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: result of a placebo-controlled, double blind study. Hepatology 1997;25:1351-60
- Jiang Q, Jiang XH, Zheng MH, L-Ornithine-l-aspartate in the management of hepatic encephalopathy: a meta-analysis. J Gastroenterol Hepatol 2009;24:9-14
- Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 2006;44:788-94
- Als-Nielsen B, Gluud LL, Gluud C. Benzodiazepine receptor antagonists for hepatic encephalopathy. Cochrane Database Syst Rev 2004;(2):CD002798
- Barbaro G, Di Lorenzo G, Soldini M, Flumazenil for hepatic encephalopathy grade III and IVa in patients with cirrhosis: an Italian multicenter double-blind, placebo-controlled, cross-over study. Hepatology 1998;28:374-8
- Riggio O, Efrati C, Catalano C, High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology 2005;42:1158-65
- Boixadera H, Tomasello A, Quiroga S, Successful embolization of a spontaneous mesocaval shunt using the Amplatzer vascular plug II. Cardiovasc Intervent Radiol 2010. (In press)
- Maleux G, Heye S, Verslype C, Management of transjugular intrahepatic porto-systemic shunt induced refractory hepatic encephalopathy with the parallel technique: results of a clinical follow-up study. J Vasc Interv Radiol 2007;18:986-92
- Bureau C, Garcia-Pagan JC, Otal P, Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology 2004;126:469-75
- Randolph C, Hilsabeck R, Kato A, Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int 2009;29:629-35
- Kircheis G, Wettstein M, Timmermann L, Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 2002;35:357-66
- Romero-Gómez M. Critical flicker frequency: it is time to break down barriers surrounding minimal hepatic encephalopathy. J Hepatol 2007;47:10-1
- Romero-Gómez M, Córdoba J, Jover R, Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology 2007;45:879-85