Abstract
While antibiotic resistance has grabbed the headlines and the attention of the pharmaceutical industry, the lack of susceptibility of biofilms formed both on animate and inanimate surfaces deserve greater attention from the industry, medical practitioners and regulators. The current literature tells us that the inherent tolerance to antibiotics demonstrated by antibiotic-sensitive organisms when grown as a biofilm clearly identifies a major disconnect between our current practices in antimicrobial development, diagnostics and efficacy in patient treatment. A paradigm shift is required in the way we utilize conventional antimicrobials and in the way we screen for next-generation antibiotics with efficacy to treat biofilms associated with chronic, recurrent and device related infections. This paradigm shift must not only take place in industry but also in how drugs are brought to the marketplace for acceptance.
1. Background
Simply typing ‘antibiotic resistance’ into pubmed.gov (as of December 2009) instantly brought forward 114,358 papers, of which 12,989 were review articles in the field. It has been over two decades since the phrase ‘post-antibiotic era’ was coined over the concern of the return to life without antibiotics for the treatment of infectious diseases. Despite these concerns, the pipeline of new antibiotics remains essentially empty and, furthermore, interest in discovering new antimicrobials by the ever-shrinking base of major pharmaceutical companies seems nonexistent. In fact, it would appear that the responsibility of identifying next generation antimicrobials has become the bailiwick of small start-up companies, ever hoping to be absorbed by ‘big-pharma’ to bring their products to marketplace. The existing pessimism regarding the development of next-generation antibiotics has led to increased interest in alternative approaches to treatment of infections, such as a renewed interest in phage therapies Citation[1].
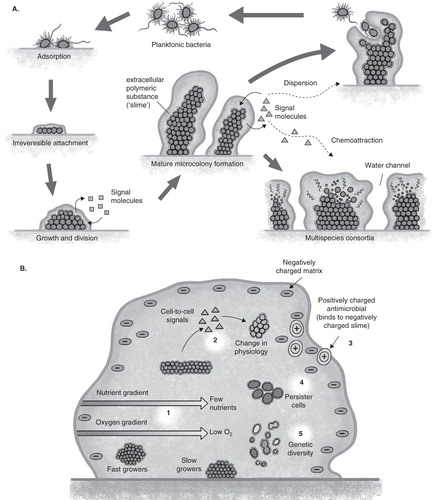
Too often ignored in the above-stated scenario is the additional complexity of treatment of chronic, recurrent and device related infections that are associated with biofilms. Biofilms can form on inanimate surfaces of medical devices, such as catheters, ventilators or orthopedic implants, or on biotic surfaces, as in the example of endocarditis or osteomyelitis. Biofilms are adherent or clustered bacteria growing as microcolonies within an exopolymeric matrix () that typically fail to respond to antibiotic treatment even when the isolate as a planktonic population is susceptible to antibiotics by minimal inhibitory concentration (MIC) testing. This lack of correlation between in-vitro MIC susceptibility testing and patient outcome in biofilm-related infections has long been recognized Citation[2]. Tolerance Citation[3], the ability of biofilms of even susceptible planktonic cells to withstand if not grow in the presence of the antibiotic, is believed to result from multifactorial mechanisms Citation[4]. These include physical properties of the biofilm, such as the surrounding matrix that can act as a barrier to antibiotic penetration as well as in the establishment of gradients of nutrients and oxygen that establish unique microenvironments within the biofilm that can select for phenotypic variants within the population that display altered antimicrobial susceptibility (). The sum of the total of the adaptive properties of a biofilm renders them tolerant to a broad range of antimicrobials and may even contribute to the development of classic antibiotic resistance through the exposure of biofilms with sub-lethal doses of drug over extended periods of time allowing them to develop and/or spread genetic changes leading to antibiotic resistance.
Figure 1. A. Growth and development of biofilms. In the center is seen the microcolony formation seen in biofilms. Lower right demonstrates polymicrobial biofilms formed through specific cell–cell signaling and attraction. Upper right demonstrates the mechanism of biofilm spread where cells become motile, swim away as a planktonic population and following to the left go through a cycle of reversible adherence, tight adherence and microcolony formation again under regulation of specific cell–cell communication. B. Multifactorial mechanisms that contribute to antibiotic tolerance developed within a biofilm.
2. The paradigm shifts
The successful treatment of biofilm infections is going to require us to rethink many aspects of how we approach antimicrobial therapy, including: how we select and use our existing antibiotics, how we both select and develop next-generation antibiotics and diagnose appropriate use of these compounds and, finally, how to deal with issues of safety and regulation of these new compounds.
2.1 Selecting biofilm-effective antimicrobials
Although it may seem self-evident, one major paradigm shift that needs to take place in the selection of antibiotics to treat biofilms is the way that we select appropriate antibiotics for treatment. It is now well established that biofilms are typically more tolerant to antibiotics (this may be up to a 1000-fold greater) than the same organism grown in suspension culture Citation[5]; therefore, if we are determining susceptibility data for antibiotic use in the treatment of biofilm infections, the assay must be done against biofilms. As mentioned earlier, there appears to be no correlation between MIC testing and patient outcomes in treating biofilm disease Citation[2], and this observation was again recently supported by the results of a comprehensive randomized, double-blind trial that demonstrated that planktonic MIC assays of synergy effects of antibiotic combinations for the treatment of cystic fibrosis patients was of no benefit to the outcome Citation[6], whereas biofilm susceptibility testing was shown to provide meaningful data on the treatment of biofilms Citation[7].
2.2 Making better use of existing antibiotics
Can we develop strategies for better treatment of biofilm infections using our current arsenal of antibiotics? A review of the literature focused on the potential use of combinations of existing antibiotics to treat biofilms in vitro, in animal models or in patients points to a number of exciting possibilities for the future Citation[7,8]; however, the complexity of these studies resulting from the array of different assays methods used to assess antibiotic efficacy and to define outcomes makes interpretation of these results problematic. There are a number of lessons to be learned: i) there will probably be no magic bullet combination that will be effective against all patient isolates of a given specific species as biofilms; a lesson we have already learnt from planktonic studies; ii) that synergy will be seen between unexpected drug combinations for which we will have no logical explanation of the mechanism, and not between compounds that one might expect to work together (); and, finally, iii) standardized assays for antibiotic synergy against biofilms must be developed. It must also be pointed out that in some cases synergies will not be limited to different antibiotic combinations, but may include combinations of antibiotics, biocides, metals, enzymes or other environmental components such as surfactants or quorum sensing inhibitors Citation[9-11].
Table 1. Susceptibility of a Pseudomonas aeruginosa cystic fibrosis isolate as a planktonic and biofilm culture to individual or combinations of antibiotics at breakpoint levels in the bioFILM PA™ Assay.
2.3 Next-generation antibiotics against biofilms
In keeping with the conceptual ideas presented above, it is obvious that in the search for new antibiotics efficacious in treating biofilms appropriate screening must be targeted against biofilms. It is not unreasonable to believe that within the enormous candidate antibiotic libraries developed through combinatorial chemistry compounds may already exist that were disregarded due to poor MIC results but may have better activity against biofilms. In fact, these may be strain or organism specific or show activity only as synergistic effectors.
A second issue in the development of next-generation antimicrobials is, once we get the screening right, what do we screen? The synergies seen in the use of existing antimicrobials suggest that our basic premise of a single, active compound with long-term antimicrobial benefit might be flawed. Do we need to look at the complexity of naturally produced antibiotics and how they function in nature Citation[12] to see if they contain adjuvant-like components that might themselves not show antimicrobial activity but which may contribute to the enhanced activity of the active compound, produce efficacy against biofilms or reduce the ability or rate of development of antibiotic resistance?
2.4 Regulatory issues
The establishment of new diagnostic assays for sensitivity will require acceptance by regulatory agencies; however, these may be able to be grandfathered as equivalents of MIC testing. There will also be more stress placed of regulatory agencies by the development of antibiotics that may contain multiple components or active agents, as this is moving into new territory for everyone. It will take patience and support to help create a new culture of cooperation and respect as this process proceeds.
3. Conclusion
As antibiotic resistance continues to be the main focus of new antibiotic development, it must be understood that tolerance to antibiotics seen in biofilms represent a major issue to treatment of infections and likely contributes to the development of antibiotic resistance. It is necessary to develop and implement new diagnostics and assays to select appropriate antibiotics for the treatment of biofilm-associated disease.
4. Expert opinion
The continued production of antimicrobials, in the face of evolutionary pressure, would suggest their continued value in nature. Yet the selection and use of antibiotics in the treatment of disease has resulted in levels of resistance that threaten the functionality of our antibiotic arsenal. The disconnect between bacterial susceptibility to antibiotics in MIC tests of planktonic bacteria and the inherent tolerance of bacteria in the biofilm mode of growth has contributed to the development and spread of antibiotic resistance in bacteria. As we develop the next-generation antimicrobials we need to consider the synergies now being reported between antibiotic and other antibiotics or environmental factors that may serve as adjuvants to antibiotic activity in the treatment of biofilms. Future development of antibiotics must involve screening against biofilms and what factors in naturally derived antimicrobial ferments may be needed to enhance efficacy of the defined active compounds against biofilms.
Declaration of interest
H Ceri was the co-inventor and holds several patents on the MBEC™ Assay Technology (Calgary Biofilm device). He was a co-founder and owns stock in Innovotech but has no direct activity in the operation of the company, nor does he derive remuneration from the company. ME Olson is the director of research and development of, and owns stock in Innovotech. He was a co-inventor of the MBEC Assay Technology (Calgary Biofilm device) and is a co-founder of Innovotech.
Notes
Bibliography
- Luna CM, Bruno D. Role of bacteriophage in the preparedness for fighting against pneumonia in the postantibiotic era. Crit Care Med 2009;37:779-80
- Smith AL, Fiel SB, Mayer-Hamblett N, Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration-lack of association in cystic fibrosis. Chest 2003;123:1495-502
- Harrison JJ, Ceri H, Turner JR. Multimetal resistance and tolerance in microbial biofilms. Nat Rev Microbiol 2007;5:928-38
- Harrison JJ, Turner RJ, Marques LR, Ceri H. Biofilms: a new understanding of these microbial communities is driving a revolution that may transform the science of microbiology. Amer Sci 2005;93:508-15
- Ceri H, Olson ME, Stremick C, The calgary biofilm device: a new technology for the rapid determination of antibiotic susceptibility of bacterial biofilms. J Clin Microbiol 1999;37:1771-76
- Aaron SD, Vandemheen KL, Ferris W, Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: a randomized, double-blind, controlled clinical trial. Lancet 2005;366:463-71
- Keays T, Ferris W, Vandemheen KL, A retrospective analysis of biofilm antibiotic susceptibility testing: a better predictor of clinical response in cystic fibrosis exacerbations. J Cyst Fibros 2009;8:122-7
- Steenbergen JN, Mohr JF, Thorne GM. Effects of daptomycin in combination with other antimicrobial agents: a review of in vitro and animal model studies. J Antimicrobial Chemother 2009;64:1130-8
- Harrison JJ, Turner RJ, Joo DA, Copper and quaternary ammonium cations exert synergistic bactericidal and anti-biofilm activity against Pseudomonas aeruginosa. Antimicrobial Agents Chemother 2008;52:2870-81
- Rivardo F, Martinotti MG, Turner RJ, Ceri H. The activity of silver against Escherichia coli biofilms is increased by lipopeptide biosurfactant. Can J Micrbiol 2010;56 (In press)
- Pan J, Ren D. Quorum sensing inhibitors: a patent overview. Expert Opin Ther Pat 2009;19:1581-601
- Aminov RI. The role of antibiotics and antibiotic resistance in nature. Environ Microbiol 2009;11:2970-88
