Abstract
Therapeutic options for treatment-experienced HIV-infected patients have been limited. The DUET trial evaluated the use of etravirine, a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), or placebo, in 1203 treatment-experienced, HIV-infected patients. Eligible patients had to have evidence of NNRTI and protease inhibitor resistance-associated mutations, and evidence of virologic failure, as defined as a plasma viral load > 5000 copies/ml on antiretroviral therapy at the time of screening. Patients in both arms received an optimized background regimen including darunavir/ritonavir. DUET demonstrated superior outcomes in virologic suppression (plasma viral load < 50 copies/ml) and clinical end points including new AIDS-defining illnesses and death, in those randomized to receive etravirine. These results were maintained at 48 weeks of follow-up. Furthermore, etravirine was shown to be safe and well-tolerated over this period. In exploratory analyses, patients in the DUET study with greater number of active agents within the background regimen were more likely to have a fully suppressive response. Taken together, the DUET results highlight the high rates of virological success that can be achieved using new active agents, such as ritonavir-boosted darunavir and etravirine, in treatment-experienced patients with underlying drug-resistant HIV infection.
1. Introduction
The pooled 48-week analysis of the DUET (TMC125 to Demonstrate Undetectable viral load in patients Experienced with antretroviral Therapy)-1 and -2 studies designed to compare etravirine with placebo in combination with a darunavir/ritonavir-containing optimized background regimens has updated the preliminary 24-week data from both trials Citation[1-3]. Treatment goals in treatment-experienced HIV-infected patients remain similar to that of treatment-naive patients, with a target of virologic suppression (plasma viral load < 50 copies/ml) Citation[4]. This has been made a realistic outcome through the advent of new antiretrovirals that either target novel sites within the viral lifecycle (enfuvirtide, raltegravir and maraviroc) or are active despite resistance to first-generation antiretroviral agents Citation[5-9]. The ‘next-generation’ antiretroviral agents include darunavir, a protease inhibitor active in the setting of underlying resistance to other protease inhibitors, and etravirine, a new non-nucleoside reverse transcriptase inhibitor (NNRTI), which retains activity against viruses demonstrating resistance to efavirenz and nevirapine Citation[10,11]. The DUET studies assessed the clinical efficacy of etravirine compared with placebo, given in combination with a darunavir/ritonavir-containing optimized background regimen among treatment-experienced patients with documented HIV drug resistance and failing ongoing antiretroviral therapy. DUET represents the first antiretroviral clinical trial in experienced patients to offer combination therapy including the coadministration of two next-generation agents.
2. Results of the pooled DUET-1 and -2 trials
2.1 Study design
The DUET-1 and -2 trials enrolled treatment-experienced adult patients for a 1:1 double-blind randomization of etravirine or placebo for 48 weeks. To be considered eligible for recruitment, patients had to have at baseline evidence of NNRTI resistance-associated mutations, at least three primary protease inhibitor mutations, and had to have evidence of virologic failure, as defined as a plasma viral load > 5000 copies/ml on therapy. The pooled trials randomized 1203 patients to etravirine 200 mg twice daily or similarly administered placebo. Patients in both arms also received a background regimen consisting of darunavir/ritonavir, at least two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and optional enfuvirtide, as selected by the investigator. Randomization was stratified by screening viral load (< 30,000 copies/ml or ≥ 30,000 copies/ml), and the use of enfuvirtide (naive vs re-used/not used), where the use of de novo enfuvirtide was limited to 40% of enrolled patients.
The primary end point of the study was the proportion of patients achieving virologic suppression with viral load < 50 copies/ml at week 24, while secondary end points included proportion of patients with viral load < 400 copies/ml, CD4 cell count change and assessment of safety and tolerability of etravirine.
2.2 Study results
Of 1203 patients randomized, 447 patients in the etravirine arm and 361 patients in the placebo arm completed 48 weeks of therapy. Approximately 90% of participants were male, and 38 and 36% in the etravirine and placebo arm respectively had a screening viral load > 100,000 copies/ml. In total, 54% of patients in the etravirine group and 55% of those in the placebo arm had a regimen with less than two active agents as determined by phenotypic sensitivity score (PSS). Overall 46% of patients were prescribed enfuvirtide as a component of the background regimen, with 26% being enfuvirtide-naive.
Patients receiving etravirine were significantly more likely to achieve viral load < 50 copies/ml at 48 weeks than placebo (61 vs 40%, respectively; p < 0.0001). In patients who achieved the primary end point of viral load < 50 copies/ml at 24 weeks, suppression was maintained in 92 and 89% of patients in the etravirine and placebo arms respectively. When analyzed according to de novo enfuvirtide use, 71% in the etravirine arm had viral load < 50 copies/ml at week 48 compared with 59% in the placebo arm (p = 0.019). Amongst those who did not use or re-used enfuvirtide, 57 versus 33% in the etravirine and placebo arms respectively had viral load < 50 copies/ml at week 48.
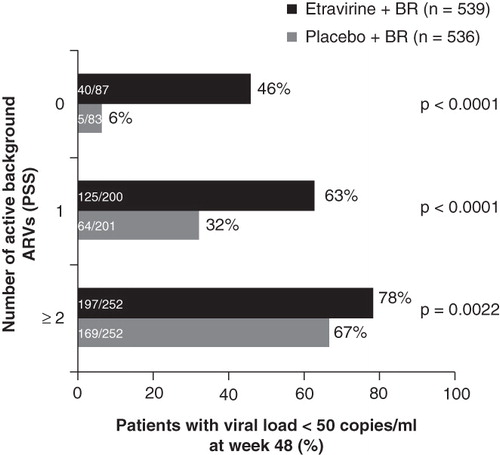
In exploratory analyses, virologic response increased in those with increased number of active agents in the regimen (). Additional factors associated with virologic response included lower baseline viral load and higher proportion of adherence. Baseline etravirine fold-change, as determined by a weighted scoring system developed to assess the effect of 17 additional etravirine resistance-associated mutations after analysis of 24-week DUET data Citation[12], was also a significant predictor of virologic response in those receiving etravirine. Highest responses in DUET were observed in those with an etravirine clinical cutoff of ≤ 3 (74% for the overall treatment arm) compared with a clinical cutoff of > 13 (48% for the overall treatment arm).
Figure 1. Response to etravirine and background regimen (BR) stratified by number of active background antiretrovirals by phenotypic sensitivity score (PSS).
Clinically, those in the etravirine arm had lower proportion of new AIDS-defining illness or death (6 vs 10%, p = 0.0408), and had a longer duration before development of this endpoint: 59 versus 45 weeks (p = 0.0108). CD4 cell count increases occurred in both arms, but was greatest in the etravirine arm (98 vs 73 cells/μl, p = 0.006). Etravirine remained safe and well tolerated at week 48, with no new concerns identified compared with week 24. The most frequent adverse event associated with etravirine remained rash (19 vs 11%, p = 0.0001); however, the majority of cases were mild to moderate in nature. Overall, 2.2% of those treated with etravirine discontinued therapy because of the rash. Of note, there was no apparent relationship between previous history of NNRTI-associated rash and risk of etravirine-related rash, and no relationship was found between baseline CD4 cell count and risk of etravirine-related rash. The incidence of rash was noted to be higher in women than in men (30 vs 18%). The overall incidence of neuropsychiatric symptoms was low at 17% in the etravirine arm and 20% in the placebo arm, a nonsignificant difference.
3. Significance of the results
The results from the DUET trials demonstrated high rates of virologic suppression derived from etravirine given in combination with a darunavir/ritonavir-containing optimized background regimen among treatment-experienced patients with documented HIV drug resistance and failing ongoing antiretroviral therapy. These results are maintained at 48 weeks. The results of this study also confirm the effectiveness of etravirine in individuals with underlying NNRTI resistance mutations, confirming earlier in vitro results Citation[11]. Of note, the presence of the K103N mutation, a common NNRTI resistance mutations arising from exposure to first-line NNRTIs did not affect response. However, it should be noted that the contribution of etravirine is increasingly compromised with increasing levels of baseline NNRTI resistance-associated mutations, and this should be evaluated before selection of etravirine for a salvage regimen.
The results of the 48-week analysis also demonstrate a significant clinical benefit accompanying virologic suppression, with fewer individuals in the etravirine arm demonstrating new AIDS-defining illnesses or death, an outcome that was not statistically different between arms at week 24 Citation[2].
The results of the trial must also be interpreted with the recognition that darunavir/ritonavir was a component of the background regimen for both study arms, and the overall virologic suppression rate amongst those in the placebo arm is relatively high, and is similar to the outcome for those receiving darunavir/ritonavir in the POWER 1 and 2 trials Citation[9]. Virologic suppression to < 50 copies/ml amongst the placebo arm in the POWER trials was only 10% after 48 weeks Citation[9]. Overall, the safety profile over 48 weeks remained stable, with similar adverse event profiles between groups with respect to neuropsychiatric symptoms (a common feature of the first-line NNRTI efavirenz). There was, however, a significantly higher risk of rash amongst those treated with etravirine. This was not associated with features of more severe toxicity, and was not linked to baseline CD4 cell count, as is the case for nevirapine-associated toxicity. There was an interesting association between development of rash and female gender, and it should be noted that women made up only 10% of the study population.
4. Expert opinion
The results of the DUET trials are an important development in clinical management of treatment-experienced HIV-infected patients. Patients initiating antiretroviral therapy early in the treatment era have experienced therapeutic failure, driven by exposure to monotherapy- and dual therapy-based regimens Citation[13]. Patients with evidence of virologic failure after first-line regimens in the early treatment era have high rates of detectable resistance mutations Citation[14] with likely compromise of second-line treatment options. For those with multidrug-resistant virus, treatment options before 2003 were limited to recycling of drug classes and multiple-drug (mega-HAART) rescue therapy, with concomitant pill burden and toxicity Citation[15]. The development of new drug classes and ‘next-generation’ agents within existing drug classes active despite resistance to first-line agents has revolutionized therapy for treatment-experienced patients.
The first novel agent studied in this population was the fusion inhibitor, enfuvirtide. The TORO studies randomized triple-class experienced patients to either an optimized background regimen alone or an optimized regimen and enfuvirtide Citation[5,16]. At 48 weeks 18.3% of those in the enfuvirtide arm were able to maintain a viral load of < 50 copies/ml, compared with 7.8% in the control arm () Citation[16]. The POWER trials evaluated the use of boosted darunavir in patients with more than one primary protease inhibitor resistance mutation to optimized regimen and darunavir versus a control protease inhibitor. At 48 weeks 45 versus 10% of patients receiving darunavir/ritonavir versus control protease inhibitor were able to maintain virologic suppression Citation[9]. These results are similar to results obtained from the MOTIVATE studies, evaluating the use of the CCR5 antagonist, maraviroc Citation[7] and the new integrase inhibitor raltegravir (BENCHMRK) () Citation[6].
Table 1. Comparison of 48-week analysis of recent randomized trials for treatment-experienced HIV-infected patients.
The 48-week analysis of DUET confirms the durability of response to etravirine therapy in combination with ritonavir-boosted darunavir, demonstrates no new safety concerns and again highlighted the observation that better responses are seen in those with more active agents in the background regimen (). This well-established observation received additional support from the DUET studies, which highlighted the efficacy of combining two new next-generation agents in the therapy of treatment-experienced patients. The ability to construct a fully active regimen for these patients has now been demonstrated in the ANRS TRIO study, where 103 patients with multidrug-resistant virus received combination etravirine, darunavir/ritonavir and raltegravir Citation[17]. At 48 weeks 86% (95% CI 80 – 93%) had achieved a viral load < 50 copies/ml, a proportion rivaling that seen in treatment-naive individuals.
The use of combination therapies for treatment-experienced patients has now become the standard of care in the developed world, and the DUET trial results played a significant role in this evolution.
Declaration of interest
J Montaner receives support from the National Institue of Drug Abuse, and was a member of the Duet 2 study group.
Notes
Bibliography
- Katlama C, Haubrich R, Lalezari J, Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS 2009;23:2289-300
- Madruga JV, Cahn P, Grinsztejn B, Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007;370:29-38
- Lazzarin A, Campbell T, Clotet B, Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007;370:39-48
- Hammer SM, Eron JJ Jr, Reiss P, Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 2008;300:555-70
- Lazzarin A, Clotet B, Cooper D, Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med 2003;348:2186-95
- Steigbigel RT, Cooper DA, Kumar PN, Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008;359:339-54
- Gulick RM, Lalezari J, Goodrich J, Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 2008;359:1429-41
- Katlama C, Esposito R, Gatell JM, Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS 2007;21:395-402
- Clotet B, Bellos N, Molina JM, Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 2007;369:1169-78
- De Meyer S, Azijn H, Surleraux D, TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother 2005;49:2314-21
- Andries K, Azijn H, Thielemans T, TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother 2004;48:4680-6
- Vingerhoets J, Tambuyzer L, Azijn H, Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled Phase III clinical studies. AIDS 2010;24(4):503-14
- Ledergerber B, Lundgren JD, Walker AS, Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 2004;364:51-62
- Recsky MA, Brumme ZL, Chan KJ, Antiretroviral resistance among HIV-infected persons who have died in British Columbia, in the era of modern antiretroviral therapy. J Infect Dis 2004;190:285-92
- Montaner JS, Harrigan PR, Jahnke N, Multiple drug rescue therapy for HIV-infected individuals with prior virologic failure to multiple regimens. AIDS 2001;15:61-9
- Nelson M, Arasteh K, Clotet B, Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr 2005;40:404-12
- Yazdanpanah Y, Fagard C, Descamps D, High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis 2009;49:1441-9
