Abstract
Importance of the field: Androgenetic alopecia affects up to 80% of males by the age of 80. The synonym ‘male-pattern hair loss’ highlights the fact that hair loss occurs in a defined and reproducible pattern. Hair loss results in reduced self esteem, loss of confidence and anxiety in affected men. An effective treatment for hair baldness would be desirable.
Areas covered in this review: In androgenetic alopecia, hair follicles undergo progressive miniaturization. Genetic factors and androgens play a major role in the pathogenesis of the disease. Polymorphism of the androgen receptor gene was first identified in association with androgenetic alopecia. Identification of new susceptibility genes on chromosomes 3q26 and 20p11 suggest that non-androgen-dependent pathways also are involved.
What the reader will gain: Topical monoxidil and oral finasteride are commonly in use and have FDA approval for the treatment of male androgenetic alopecia; dutasteride, a type I and II 5-alpha-reductase inhibitor, is on hold in Phase III trials. A combination of medical treatment and hair transplant surgery has shown superior efficacy.
Take-home message: Androgenetic alopecia is a progressive condition and although the current available treatments are effective in arresting the progression of the disease, they allow only partial regrowth of hair at its best. Early treatment achieves the best desirable outcome.
1. Introduction
Androgenetic alopecia (AGA), or male-pattern hair loss (MPHL), is a genetically determined progressive process that causes a gradual conversion of terminal hair into vellus hair. The prevalence increases with advancing age; however, the age of onset and rate of progression are variable Citation[1].
The morbidity of MPHL is predominantly psychological and varies from person to person. Affected men find themselves less attractive and older looking than non-balding men Citation[2]. A study found that women and non-balding men have a negative perception towards bald men and that this is more or less the same as the negative perception bald men have of themselves Citation[3].
Minoxidil lotion and finasteride tablets are both FDA approved for the treatment of hair loss. Finasteride is a 5-alpha-reductase type II inhibitor that reduces conversion of testosterone to the more bioactive metabolite dihydrotestosterone (DHT). Dutasteride is a dual type I and type II 5-alpha-reductase inhibitor and has shown superior efficacy to finasteride in Phase II trials; however, Phase III trial data are lacking. These agents are effective at arresting hair loss but are able to achieve only limited hair regrowth. They are most useful in the management of early androgenetic alopecia.
Hair transplant remains the only viable treatment for advanced hair loss, but this requires donor occipital hairs. Newer agents such as prostaglandin analogues, gene therapy and angiogenic growth factors have not been shown to have a place in patient management but are targets for future research.
2. Pathophysiology
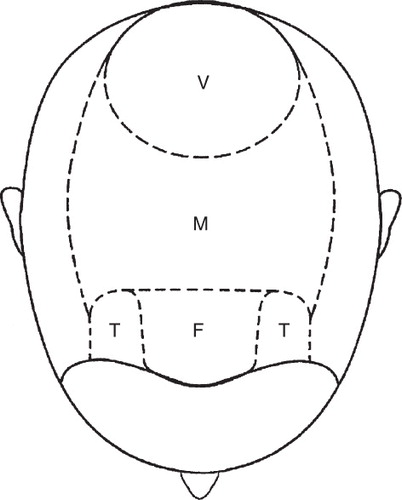
In androgenetic alopecia, large terminal hairs are shed and are replaced by small vellus hairs. Three areas of the scalp are affected preferentially: temples, vertex scalp and mid-frontal scalp (). Within these areas, the process is strictly patterned. Bitemporal hair loss starts at the anterior hair line and moves posteriorly over the scalp. Hair loss over the vertex scalp begins centrally and radiates outwards circumferentially. Over the mid-frontal scalp, hair follicle miniaturization leads to a pattern of hair loss reminiscent of a Christmas tree Citation[4].
As the three zones are not affected equally, there are clinical variations in the pattern of hair loss, with some men balding more to the front and others more over the crown. Asian men tend to develop diffuse thinning over the mid-frontal zone with the frontal hair line, temples and vertex only minimally affected Citation[5]. This produces a pattern reminiscent of that described in women by Ludwig Citation[6].
The volume of the dermal papilla is directly related to the size of the emerging hair fibre Citation[7]. Miniaturization involves reduction in the volume of the dermal papilla and this is due to both a reduction in the extracellular matrix and the total number of cells Citation[8]. Cells are thought to travel out of the dermal papilla and into the dermal sheath during catagen, and to re-enter the dermal papilla from the dermal sheath during anagen Citation[9]. A net reduction in the number of cells re-entering the dermal papilla will result in miniaturization. The factors that regulate cell traffic into and out of the dermal papilla are poorly understood.
Although miniaturized hairs are also seen in alopecia areata, that condition is potentially fully reversible. By contrast, androgenetic alopoeica is only partially reversible at its best Citation[10]. Early treatment is associated with superior regrowth of hair in androgenetic alopecia Citation[11]. The mechanism for difference of regrowth of hair in these two conditions is poorly understood, but preliminary data suggest a role for the arrector pili muscle. Horizontal scalp biopsy sections stained with Van Giessen stain have been used to reconstruct the arrector pili muscle in three dimensions in relation to the hair follicle Citation[12]. Connection of the arrector pili muscle and the hair follicle is preserved in alopecia areata but is lost in androgenetic alopecia Citation[12]. Interaction between the mesenchymal muscle cells and the epithelial stem cells in the bulge could be a requirement for reversal of miniaturization.
2.1 Hair cycle dynamics
Hair is lost and replaced cyclically. Follicles undergo corresponding cyclical phases of growth, involution, quiescence and regeneration. The growth phase (anagen) last for 3 – 5 years. As hair elongation is relatively constant at 1 cm per month, the duration of the growth phase is the primary determinant of the final hair length. At the end of anagen, the involutional phase is known as catagen; this lasts for few weeks. The period of hair follicle quiescence that follows catagenm, lasting approximately 3 months, is known as telogen Citation[13]. Hair follicle regeneration occurs in the first week or so of anagen and, once regenerated, the anagen phase continues until the hair reaches its final (possibly predetermined) length.
A number of molecular signals including growth factors, nuclear receptors, cytokines and intracellular signalling pathways are involved in controlling the hair cycle. Growth factors such as insulin-like growth factor (IGF-1), hepatocyte growth factor, keratinocyte growth factor and vascular endothelial growth factor (VEGF) promote the anagen phase of the hair cycle Citation[14]. In addition to the hair follicle miniaturization that results in thin fibres in androgenetic alopecia, a reduction in anagen duration leads to shorter hair length, while an increase in telogen duration delays regeneration. This results in hairs so short and fine that they fail to achieve sufficient length to reach the surface of the scalp, resulting in increased number of empty pores Citation[15].
2.2 Genetics in androgenetic alopecia
Androgenetic alopecia is familial. Twin studies identified heredity as accounting for around 80% of the predisposition to baldness Citation[16]. Polygenic inheritance best accounts for the range of clinical phenotypes and increased risk with increased number of affected family members Citation[17]. Ellis et al. observed that the fathers of 81.5% of affected men have baldness that is higher than that that can be explained by autosomal dominant inheritance Citation[18].
Polymorphism of the androgen receptor (AR) gene was first identified in association with androgenetic alopecia Citation[19], and has subsequently been confirmed by numerous other investigators in different patient cohorts. Functional confirmation of the importance of the androgen receptor gene in androgenetic alopecia comes from the recognition that men affected by Kennedy disease, a familial neuromuscular disorder associated with a functional alteration of the androgen receptor, have a reduced risk of developing androgenetic alopecia Citation[20].
The observation of AR gene polymorphism in the majority of non-balding men suggests that other factors are necessary to cause the hair loss. Ellis et al. suggested that abnormality of the AR gene is necessary but not sufficient to cause the phenotype Citation[19]. The X chromosomal location of the AR gene indicates that the maternal line is the major inheritance of androgenetic alopecia in men. However, family studies have shown resemblance of hair loss between fathers and sons, which cannot be explained by AR gene mutations. This suggests that other autosomal genes might also contribute to the phenotype.
Hillmer et al. have investigated towards identification of new susceptibility genes in AGA. In a genome-wide scan and fine-mapping linkage study performed on 95 families in which at least two brothers had early-onset AGA and both parents were available, Hillmer et al. found strong evidence for an AGA susceptibility locus on chromosome 3q26 Citation[21]. This study could not confirm or rule out the relevance of chromosomes 11q22-q24, 18p11-q22 and 19p13-q13 in causing AGA. Another genome-wide association study done by Hillmer et al. found that a highly significant association on chromosome 20p11, suggesting that the 20p11 locus has a role in a yet-to-be-identified androgen-independent pathway Citation[22].
The role of the 5-alpha-reductase enzyme in male androgenetic alopecia (MAA) is evident by the part it plays in the metabolism of testosterone to DHT and the effect of 5-alpha-reductase inhibitors in treating hair loss. Genetic association studies of the 5-alpha-reductase genes SRD5A1 on chromosome 5 and SRD5A2 on chromosome 2, done using dimorphic intragenic restriction fragment length polymorphisms in 828 families, failed to show an association between these genes and MAA Citation[23].
The cytochrome p450 alpha-aromatase enzyme has also been found to contribute to androgenetic alopecia. Aromatase catalyses the conversion of testosterone to estradiol and diminishes intrafollicular testosterone. There is difference in expression of aromatase in balding and non balding scalp Citation[24]. Yip et al. suggest that the aromatase gene (CYP19A1) might predispose to hair loss in women Citation[25]. However, case-control studies have failed to demonstrate a difference of the involvement of aromatase gene in causing andogenetic alopecia Citation[26].
2.3 Androgens and androgenetic alopecia
The role of androgen in male-pattern hair loss is well established. American anatomist James Hamilton observed that castrated males did not develop androgenetic alopecia unless they were treated with testosterone Citation[27]. Testosterone is the main circulating androgen and the tissue effects of androgens are mediated by biding trough to the intracellular androgen receptor Citation[28]. Evidence that patients with androgen insensitivity syndrome, caused by a mutation in the androgen receptor gene, do not go bald suggests that androgenetic alopecia is induced by the effect of testosterone on the androgen receptor Citation[29].
In most body sites, hair growth is mediated by 5-alpha-dihydrotestosterone (DHT), a potent metabolite of testosterone that has a several-fold higher sensitivity to the androgen receptor. The conversion of testosterone to DHT is catalysed by 5-alpha-reductase. There are two isoforms of the enzyme (type 1 and 2), and both are capable of producing DHT from testosterone, although they have different biochemical and distinct tissue-specific expression patterns Citation[30]. Higher concentrations of 5-alpha-reductase activity have been observed in bald scalps.
The important role of 5-alpha-reductase activity in androgenetic alopecia is supported by the absence of temporal regression and baldness in cases of 5-alpha-reductase deficiency caused by mutations in the 5-alpha-reductase gene Citation[31]. The effect of 5-alpha-reductase inhibitors in halting the progression of hair loss in men further supports the role of 5-alpha-reductase in androgenetic alopecia Citation[32].
Individual susceptibility to hair loss and its severity is also determined by local factors. A highly ordered intrinsic susceptibility of hair follicles to androgen gives a specific pattern of hair loss. Occipital hair is resistant to androgens, even when it is transplanted into the vertex. However, vertex hair continues to miniaturize when transplanted on the forearm Citation[33,34]. Itami and Inui proposed that the second-messenger system determines whether androgen causes hair growth in beard and axillary area and at the same time miniaturizes terminal hair on the scalp Citation[35].
3. Clinical features
The essential feature of male androgenetic alopecia is patterned hair loss. The progression of the hair loss occurs in the orderly manner described by Hamilton and Norwood Citation[36,37]. If not treated in the early stage, hair loss progresses, leaving a completely hair less scalp, particularly on the crown; occipital hair is usually preserved (). The onset of hair loss and the rate of progression varies from person to person.
Figure 2. Progressive hair loss in the crown in advancing stages, with relative sparing of the occipital hair.
Close examination with a hand-held epiluminescent microscope (dermatoscope) demonstrates that scalp hairs exist as compound follicles with two to five terminal hairs emerging from a single pore. In the balding scalp, the terminal hairs within these compound follicles are progressively replaced by finer and shorter vellus hairs such that only one or two terminal hairs can be seen emerging from a single pore in affected areas (). A reduction in the volume of hair can be noticed when the number of terminal hairs per follicular unit decreases; however, baldness becomes visible only when all the terminal hairs are miniaturized. Dermatoscopy can demonstrate hair diameter diversity due to progressive miniaturization before clinical baldness is visible and is characteristic of androgenetic alopecia Citation[38]. In addition, a reduction in the number of terminal hairs in a single pore is a useful feature in early diagnosis of androgenetic alopecia in patients presenting with increased hair shedding. By contrast, compound follicles persist on the occipital scalp even in advance stages of male-pattern hair loss.
4. Treatments currently in use
4.1 Minoxidil
Oral minoxidil has been used to treat hypertension since the 1960s Citation[39]. Hypertrichosis as a consequence of minoxidil treatment was observed shortly thereafter and has been said to occur in 100% of users Citation[40-42]. These observations led to the development of topical minoxidil as a treatment for hair loss Citation[43]. It was approved by the FDA for the treatment of male androgentic alopecia in 1984. Both 2 and 5% solutions are currently in use for treatment in male; the 5% monoxidil solution has shown higher efficacy than the 2% solution Citation[44]. New 5% minoxidil foam is now available and reported to be as effective as the solution Citation[45]. A 5-year follow-up study with topical minoxidil has shown a sustained effect Citation[46].
A number of investigators have advanced hypotheses as to the mechanism of action of minoxidil. One important hypothesis concerns its vasodilatory properties. Cutaneous blood flow was observed to increase 10 – 15 min after applying topical minoxidil Citation[47]. Upregulation of vascular endothelial growth factor (VEGF) is another important action of minoxidil that helps to maintain dermal papilla vasculature and hair growth Citation[48]. Li et al. proposed a possible mechanism for minoxidil stimulation of VEGF from experiments on dermal papilla cells Citation[49]. They suggest that binding of minoxidil to adenosine receptors A1 and A2, as well as to the sulphonylurea receptor SUR2B, activates adenosine signalling pathways and increases the release of VEGF. Over-expression of VEGF increase peri-follicular vascularization and accelerate hair growth.
The prevailing view is that minoxidil promotes hair regrowth through its action as a potassium channel opener Citation[50,51]. The effect on the cell cycle is to initiate the onset of anagen (and thereby shorten the duration of telogen) and to prolong the duration of anagen by delaying initiation of catagen. Patients commonly observe increased hair shedding a few weeks after starting therapy; this resolves spontaneously with continued treatment.
Hypertrichosis on the face and hands is a common side effect observed after topical minoxidil. Itching of the scalp, increased dandruff and erythema are commonly reported. Contact allergic dermatitis to minoxidil can occur and could be due either to minoxidil itself or, more commonly, to propylene glycol in the vehicle Citation[52]. Patch testing is worthwhile in differentiating the cause of contact dermatitis and the new minoxidil form that does not contain propylene glycol is an alternative in these patients.
4.2 Finasteride
Finasteride is a 5-alpha-reductase type II inhibitor that acts by reducing scalp DHT levels Citation[53]. Scalp biopsies before and after treatment with finasteride show a reduction of scalp DHT by 60 – 75% Citation[54]. The optimal dose of finasteride in the treatment of androgenetic alopecia is 1 mg a day. Placebo-controlled studies have shown that finasteride significantly increases hair count after 1 – 2 years of therapy and the effect is sustained after 5 years of therapy Citation[55-58]. An open, randomized, comparative study with 5% topical minoxidil and oral finasteride 1 mg a day showed significantly more hair growth in the finasteride group Citation[59].
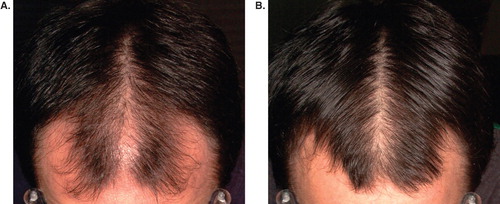
Good-quality serial photographs (pre- and post-treatment) are important for patient and clinician to make an objective assessment of the clinical response to finasteride ().
Figure 4. Photographic evaluation of treatment response to finasteride. (A) Pre-treatment; (B) post-treatment.
Decreased libido, erectile dysfunction and ejaculatory problems have been reported in around 3 – 5% of men Citation[60]. Gynecomastia, exfoliative dermatitis, testicular pain and depressive mood changes have been observed in rare cases Citation[61]. Finasteride reduces prostate-specific antigen (PSA) levels by approximately 50% and adjustments should be made when interpreting screening tests for prostate cancer Citation[62]. Finasteride also affects the rate and grade of prostate cancer. There is a 25% overall reduction in the prevalence of prostate cancer but a small relative increase in the percentage of high-grade cancers, limiting its use as a chemoprevention agent Citation[63].
4.3 Dutasteride
Dutasteride has similar characteristics as finasteride but is more potent in reducing scalp DHT. It inhibits both type I and II 5-alpha-reductase enzymes.
Dutasteride is FDA approved for the treatment of benign prostatic hyperplasia (BPH). Phase II studies in androgenetic alopecia demonstrated a dose-dependent increase in hair growth and the efficacy of finasteride 2.5 mg/day was superior to that of finasteride 5 mg/day Citation[64]. Twin studies showed that 0.5 mg dutasteride a day for 1 year resulted in significantly more hair growth than placebo Citation[65]. A single-case report showed improvement with dutasteride in a woman who had failed to show any response to finasteride Citation[66]. These findings all require confirmation in large, case-controlled studies.
Impotence, decreased libido, breast tenderness and breast enlargement and ejaculation disorders are more common with dutasteride than with finasteride.
4.4 Hair transplantation
Hair transplantation involves removal of hair from the occipital scalp and reimplantation into the bald vertex and frontal scalp. With modern techniques, graft survival in excess of 90% can be reliably achieved. Prerequisites for the procedure are stabilization of the hair loss with medical treatment and good donor hair population on the occipital hair.
The modern hair transplant technique was started in Japan in 1930s, when small punch grafts were used to cover damaged eyebrows or lashes Citation[67]. Orentreich reported on the use of autografts and proposed the term ‘donor dominance’ Citation[68] in that hair taken from the androgen-resistant occipital scalp remains androgen resistant when implanted into the androgen-sensitive bald areas of the scalp.
In 1995, Bernstein and Rassman introduced follicular unit transplantation, by which hair is transplanted in naturally occurring units of 1 – 4 hairs Citation[69]. In follicular unit transplantation, donor hair can be harvested in two different ways:
1) Strip harvesting: a strip of scalp 8 – 14 mm and 20 – 30 cm is removed from the occipital scalp under local anaesthesia and the wound sutured. The donor hair is then separated into follicular units and transplanted to the balding area. The main disadvantage of this method is that it leaves a linear scar in the donor occipital area, although this could be concealed with the remaining occipital hair.
2) Follicular unit extraction (FUE) harvesting: individual follicles of occipital hair are removed under local anaesthesia with 1 mm punch biopsies. Each unit is then reinserted on the bald areas of the scalp using a micro blade. This technique leaves no visible scars as single follicular units are removed instead of a large amount of tissue, it also takes less time to heal than strip harvesting.
5. Emerging therapeutic options
5.1 Latanoprost
The prostaglandin analogue, latanoprost, stimulates hair growth but the precise mechanism of action is unknown. Lengthening of eyelashes and eye brows has been observed when latanoprost is used topically for glaucoma Citation[70] but there is no conclusive evidence to suggest that it has efficacy in stimulating scalp hair growth.
5.2 Androgen receptor blockers
Androgen and androgen receptors play a major role in the pathogenesis of androgenetic alopecia. Androgen receptor blockers, including spironolactone, cyproterone acetate and flutamide, have demonstrated efficacy in female-pattern hair loss. When treated with either spironolactone or cyproterone acetate for 12 months, 88% of patients with biopsy-proven female pattern hair loss have shown partial improvement or no progression of the disease Citation[71]. However, a 12-month randomized trail comparing 2% minoxidil and cyproterone acetate failed to demonstrate superior efficacy of antiandrogen therapy over topical minoxidil Citation[72]. Placebo-controlled studies are necessary to confirm the efficacy of anti-androgens in female-pattern hair loss. However, feminization in males does not warrant their use in male-pattern hair loss. The use of flutamide is limited by the risk of serious, albeit rare, hepatotoxicity.
6. Combination of medical and surgical therapy
An open, randomized, parallel-group study comparing the efficacy of available medications as monotherapy or combined therapy (finasteride alone, finasteride + 2% topical minoxidil, topical minoxidil alone and finasteride + ketokonazole shampoo) showed that finasteride in combination with either topical minoxidil or ketoconazole showed significantly better hair regrowth than finasteride monotherapy, and showed no difference in the incidence of side effects. It is inferred that a combination of medications with different mechanisms of action enhances the efficacy Citation[73].
Topical minoxidil and finasteride can be useful adjuncts to hair transplant surgery for AGA. Studies have found that topical use of minoxidil in the peri-operative period could prevent the usual shedding that occurs 1 – 2 weeks after transplantation and speed the time for regrowth Citation[74]. These results were confirmed by a double-blind trial that showed that significantly less grafted hair was lost during the shedding period Citation[75]. The use of topical minoxidil as a premedication in hair transplant surgery has the advantage of stabilizing the hair loss, increasing the number of hairs in anagen and decreasing post-surgical telegon effluvium. Minoxidil should be stopped 2 – 3 days before surgery to minimize skin irritation and reduce the theoretical risk of intraoperative bleeding caused by vasodilation. Therapy should be restarted again after 1 – 2 weeks. A randomized, double-blind trial using finasteride 1 mg daily or placebo in 79 men with AGA 4 weeks before and 48 weeks after hair transplantation demonstrated that the treatment group had significant improvement from baseline, in comparison with placebo group Citation[76].
7. Expert opinion
The diagnosis of male androgenetic alopecia involves recognizing hair miniaturization in a characteristic distribution over the scalp. Miniaturization initially leads to loss of hair volume and later to bald areas on the scalp. Genetic predisposition seems to be more important than environmental factors in the aetiology. The pathogenesis involves interaction of testosterone and its more active metabolite DHT, with androgen receptors leading to changes in hair cycling.
Medical treatment involves inhibiting the of synthesis DHT using 5-alpha-reductase inhibitors such as finasteride and dutasteride or medications that alter hair cycle dynamics such as minoxidil. Finasteride has been shown to arrest hair loss in most men and to stimulate partial regrowth in around 66%. Dutasteride 2.5 mg/day appears more effective than finasteride 5 mg/day at stimulating regrowth, but Phase III clinical trials are required to confirm this. Topical minoxidil is also effective in arresting hair loss and stimulating regrowth and can be used in combination with finasteride or dutasteride to maximize regrowth.
Androgen receptor antagonists block the effect of both DHT and testosterone, and lead to hypo-androgenization, which is poorly tolerated by men. Surgical treatment utilized the principle of donor dominance, whereby the individual follicle retains its intrinsic relative propensity to balding after relocation to a different site. Hair transplantation is required to restore hair in more advanced cases.
Article highlights.
Androgenetic alopecia occurs in the presence of androgens in genetically susceptible individuals. The disease onset and progression vary from person to person.
Even though the disease is medically benign hair loss causes psychological morbidity in majority of the affected men.
Androgen receptor gene polymorphism plays a major role in the pathogenesis of androgenetic alopecia. The identification of new susceptibility genes on chromosomes 3q26 and 20p11 suggests a yet-to-be identified androgen-independent pathway.
Effective and safe medications are available for the treatment of androgenetic alopecia and prolonged continuous use is required for sustained effect.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Notes
This box summarizes key points contained in the article.
Bibliography
- Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc 2005;10:184-9
- Cash TF. The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol 1992;26:926-31
- Lee HJ, Ha SJ, Kim D, Perception of men with androgenetic alopecia by women and non balding men. Inter J Dermatol 2002;41(12):867-9
- Oslen EA. Female pattern hair loss: clinical features and potential hormonal factors. J Am Acad Dermatol 2001;45:570-80
- Lee WS, Ro BI, Hong SP, A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol 2007;57(1):37-46
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977;97:247-54
- Van Scott EJ, Ekel TM. Geometric relationaships between the matrix of the hair bulb and its dermal papilla in normal and alopecic scalp. J Invest Dermatol 1958;31:281-7
- Elliot K, Stephenson TJ, Messenger AG. Differences in the hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size androgen responses. J Invest Dermatol 1999;113:873-7
- Jahoda CAB. Cellular and developmental aspects of androgenetic alopecia. EXP Dermatol 1998;7:235-48
- Hamilton JB. Patterned loss of hair in man: types and incidence. Annal NY Acad Sci 1951;53:708-28
- Van Neste D, Fuh V, Sacchiez-Pedreno P, Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol 2000;143:804-10
- Yazdabadi A, Magee J, Harrison S, Sinclair R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. Br J Dermatol 2008;159:1300-2
- de Berker DAR, Messenger AG, Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors, Rook's textbook of dermatology. 7th edition. (Volume 4). Blackwell Publishing; 2004. pp. 63. 8-63.10
- Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc 2003;8:46-55
- Curtois M, Loussouarn G, Horseau C. Hair cycle and alopecia. Skin Pharm 1994;7:84-9
- Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. J Invest Dermatol 2003;121:1561-4
- Koster W, Happle R. The inheritance of baldness: two B or not two B? J Am Acad Dermatol 1984;11:921-6
- Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5 alpha reductase genes. J Invest Dermatol 1998;110:849-53
- Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Investig Dermatol 2001;116:452-5
- Sinclair R, Greenland KJ, Egmond S, Men with Kennedy disease have a reduced risk of androgenetic alopecia. Br J Dermatol 2007;157(2):290-4
- Hillmer AM, Flaquer A, Hanneken S, Genome-wide scan and Fine-Mapping Linkage study of androgenetic alopecia reveals a locus on chromosome 3q26. Am J Hum Genet 2008;82:737-43
- Hillmer AM, Brockschmidt FF, Hanneken S, Susceptibility variants for male-pattern baldness on chromosome 20p11. Nat Genet 2008;40(11):1279-81
- Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5 alpha reductase genes. J Invest Dermatol 1998;110:849-53
- Sawaya ME, Price VH. Different levels of 5 alpha reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol 1997;109:296-300
- Yip L, Zaloumis S, Irwin D, Gene-wide association study of the aromatase gene (CYP19A1) with female pattern hair loss. Br J Dermatol 2009;161(2):289-94
- Ellis JA, Harrap SB. The genetics of androgenetic alopecia. Clin Dermatol 2001;19:149-54
- Hamilton JB. Male hormone stimulation is prerequisite and an insitant in common baldness. AM J Anat 1942;71:451-80
- Williams GR, Franklyn JA, Physiology of the steroid-thyroid hormone nuclear receptor superfamily. Baillieres Clin Endocrinol Metab 1974;38:811-19
- Patterson MN, McPhaul MJ, Hughes IA. Androgen insensitivity syndrome. Baillieres Clin Endocrinol Metab 1994;8:379-404
- Jenkins EP, Andersson S, Imperato McGinley J, Genetic and pharmacological evidence for more than one human steroid 5-alpha-reductase. J Clin Invest 1992;89:293-300
- Imperato McGinley J. % alpha reductase 2 deficiency and complete androgen insensitivity: lesions from nature. Adv Exp Med Biol 2002;511:121-34
- Kaufman KD, Olsen EA, Whiting D, Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol 1998;39:578-89
- Orentreich N. Autografts in alopecias and other selected dermatological conditions. Ann NY Acad Sci 1959;83:463-79
- Nordstrom REA. Synchronous balding of scalp and hair bearing grafts of scalp transplanted to the skin of the arm in male pattern baldness. Acta Derm Venereol (Stockh) 1979;59:266-8
- Itami S, Inui S. Role of androgen in mesenchymal epithelial interactions in human hair follicle. J Investig Dermatol Symp Proc 2005;10(3):209-11
- Hamilton JB. Patterned hair loss in men: types and incidence. Ann NY Acad Sci 1951;53:708-14
- Norwood OT. Male pattern baldness: classification and incidence. South Med J 1975;68:1359-70
- De Lacharriere O, Deloche C, Misciali C, Hair diameter diversity: a clinical sign reflecting the follicle miniaturization. Arch Dermatol 2001;137:641-6
- Kosman ME. Evaluation of a new antihypertensive agent: minoxidil. JAMA 1980;244:73-5
- Devine Bl, Fife R, Trust PM. Minoxidil for severe hypertention after failure of other hypotensive agents. Br Med J 1977;2:667-9
- Jacobs D. Minoxidil experience in Australia 1974-1980. Med J Aust 1981;1:477-8
- Pennisi AJ, Takahashi M, Bernstein BH, Minoxidil therapy in children with severe hypertension. J Paediatr 1977;90:813-19
- Kreindler TG. Topical minoxidil in early androgenetic alopecia. J Am Acad Dermatol 1987;16:718-24
- Roberts JL. Androgenetic alopecia: treatment results with topical minoxidil. J Am Acad Dermatol 1987;16:705-10
- Rundegren J, Westin A, Kohut B. Hair growth efficacy assessment of a new topical minoxidil foam formulation in the stump-tail macaque. J invest Dermatol 2005;587:A98
- Olsen EA, Weiner MS, Amara IA, DeLong ER. Five year follow up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol 1990;22:643-6
- Wester RC, Maibach HI, Guy RH, Nowak E. Minoxidil stimulates cutaneous blood flow in human balding scalp: pharmacodynamics measured by laser Doppler velocimetry and photopulse plethysmography. J Invest Dermatol 1984;82:515-17
- Lachgar S, Charveron M, Gall Y, Bonafe JL. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol 1998;138:407-11
- Li M, Marubayashi A, Nakaya Y, Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J Invest Dermatol 2001;117(6):1594-600
- Buhl AE, Conrad SJ, Waldon DJ, Pottasium channel conductance as a control mechanism in hair follicles. J Invest Dermatol 1993;101:148s-52s
- Buhl AE, Waldon DJ, Conrad SJ, Pottasium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol 1992;98:315-19
- Friedman ES, Friedman PM, Cohen DE, Washenik K. Allergic contact dermatitis to topical minoxidil solution: atiology and treatment. J Am Acad Dermatol 2002;46:309-12
- Rhodes L, Harper J, Uno H, The effects of finasteride (Proscar) on hair growth, hair cycle stage, and serum testosterone in adult male and female stump-tail macaques (Macaca arctoides). J Clin Endocrinol Metab 1994;29:991-6
- Drake L, Hordinsky M, Fiedler V, The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad Dermatol 1999;41:550-4
- Kaufman KD, Olsen EA, Whiting D, Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol 1998;39:578-89
- Finasteride Male Pattern Hair Loss Study Group. Long-term (5-year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol 2002;12:38-49
- Stough DB, Rao NA, Kaufman KD, Mitchell C. Finasteride improves male pattern hair loss in a randomized study in identical twins. Eur J Dermatol 2002;12:32-7
- Van Neste D, Fuh V, Sanchez Pedreno P, Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol 2000;143:804-10
- Arca E, Acikgoz G, Tastan HB, An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology 2004;209(2):117-25
- Wilton L, Pearce G, Edet E, The safety of finasteride used in benign prostatic hypertrophy: a non-interventional observational cohort study in 14,772 patients. Br J Urol 1996;78:379-84
- Rihimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride-induced depression: a prospective study. BMC Clin Pharmacol 2006;6:7
- D'Amico AV, Roehrborn CG. Effect of 1 mg/day finasteride on concentrations of serum prostate-specific antigen in men with androgenic alopecia: a randomised controlled trial. Lancet Oncol 2007;8(1):21-5
- Thompson IM, Goodman PJ, Tangen CM, The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215-24
- Olsen EA, Hordinsky M, Whiting D, The importance of dual 5-alpha reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride vs. finasteride. J Am Acad Dermatol 2006;55:1014-23
- Stough D. Dutasteride improves male pattern hair loss in a randomized study in identical twins. J Cosmet Dermatol 2007;6:9-13
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol 2005;4:637-40
- Okuda S. The study of clinical experiments of hair transplantation. Jpn J Dermatolurol 1939;46:135
- Orentreich N. Autografts in alopecias and other selected dermatological conditions. Ann NY Acad Sci 1959;83:463-79
- Bernstein RM, Rassman WR, Szaniawski W, Halperin A. Follicular transplantation. Intl J Aest Restor Surg 1995;3:119-32
- Wolf R, Matz H, Zalish M, Prostaglandin analogs for hair growth: great expectations. Dermatol Online J 2003;9:7
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol 2005;152:466-73
- Vexiau P, Chaspoux C, Boudou P, Effect of 2% minoxidil vs cyproterone acetate on female androgenetic alopecia: a controlled, 12 months randomised trial. Br J Dermatol 2002;146:992-9
- Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol 2002;29(8):489-9
- Kassimir JJ. Use of topical minoxidil as a possible adjunct to hair transplant surgery. A pilot study. J Am Acad Dermatol 1987;16:685-7
- Roenigk HH, Berman MD. Topical 2% minoxidil with hair transplantation. Face 1993;4:213-16
- Leavitt M, Perez-Meza D, Rao NA, Effects of finasteride (1 mg) on hair transplant. J Dermatol Surg 2005;31:1268-76