Abstract
Importance of the field: Although trigeminal neuralgia has traditionally been considered the prime neuralgic condition in the face region, other forms of neuropathic pain are now being more frequently recognized and require recognition and a different management approach.
Areas covered in this review: This review principally covers medical management of trigeminal neuralgia; but also included is glossopharyngeal neuralgia, trigeminal neuropathic pain (atypical odontalgia) and burning mouth syndrome. Systematic reviews and guidelines will be discussed.
What the reader will gain: An update will be provided of drug therapy for these relatively rare facial pains.
Take home message: Trigeminal neuralgia continues to be best managed using anticonvulsant drugs, the primary ones being carbamazepine and oxcarbazepine; baclofen may be helpful and, of the newly emerging drugs, pregabalin has potential. Glossopharyngeal neuralgia remains managed in the same way as trigeminal neuralgia. Trigeminal neuropathic pain is probably best managed according to guidelines used for the management of neuropathic pain, which include the use of tricyclic antidepressants, gabapentin, pregabalin, duloxetine, venalafaxine and topical lidocaine. Burning mouth syndrome is a neuropathic pain managed initially with topical clonazepam and then with other neuropathic drugs. Patients need to be involved in their management.
1. Background
The most widely recognized neuropathic pain of the face is trigeminal neuralgia. Others that also occur include glossopharyngeal neuralgia, postherpetic neuralgia and the relatively newly emerging trigeminal neuropathic pain possibly the same as atypical odontalgia also need to be considered. There is also increasing evidence that burning mouth syndrome is a neuropathic pain. A group of conditions called the trigeminal autonomic cephalgias (TAC) form a distinct subgroup of neuralgiform-type pains principally affecting the first division of the trigeminal nerve. These include conditions known as cluster headache, paroxysmal hemicrania, short unilateral neuralgiform pain with conjunctival injection and tearing (SUNCT) and the more recently used terminology short unilateral neuralgiform pain with autonomic (SUNA) symptoms. The last probably encompass the more strict criteria of SUNCT Citation[1]. The TACs are not covered by this review. Looking through the International Headache Society classification Citation[2], a multitude of other neuralgias have been identified. The evidence for their existence as distinct entities is scant, and even the best-known trigeminal neuralgia has not been formally validated by case-control series Citation[3].
Several promising targets for treatment have been identified, such as sodium and calcium channels, glutamate receptors, monoamines and neurotrophic factors, and it has therefore been suggested that a more mechanism-based approach should be taken to management of these conditions Citation[4]. However, at present treatment is often insufficient when using this methodology and Finnerup et al. Citation[5] suggest that this is because we still lack valid and reliable tools to assess neuropathic pain and do not understand the mechanisms of individual conditions. The emergence of several screening tools based on verbal descriptors, with or without bedside testing, to attempt to diagnose neuropathic pain in recent years is evidence of the increasing attempts to phenotype these patients more accurately Citation[6]. These tools have rarely been used in facial pain conditions.
2. Trigeminal neuralgia
2.1 Overview
A review of trigeminal neuralgia and its treatments was last published in Expert Opinion in 2003 Citation[7]. This article updates the material in that review.
Trigeminal neuralgia is defined as a unilateral, severe, short-lasting pain of the face Citation[2,8]. The vast majority of cases are of unknown aetiology and the rarer forms are due to secondary causes such as benign and malignant tumours, demylination (such as occurs in multiple sclerosis) and rare arteriovascular malformations. Epidemiological studies that were mainly done in the US during the 1990s suggested that this condition was rare, with an annual incidence in the US of 4 – 6 per 100,000 patients, that it occurred in the 50 – 70 years age group and was more common in women than men Citation[9]. However, more recent studies carried out in research primary-care practices both in the UK and in Holland suggest that general medical practitioners are diagnosing trigeminal neuralgia much more frequently, with incidence rate of 28 per 100,000 Citation[10,11]. I suspect there may be an element of misdiagnosis and some of these cases will be dental pains or one of the TACs, as teaching on facial pain among medical practitioners is virtually non-existent and misdiagnosis is easy Citation[12]. Larger European studies looking at the burden of neuropathic pain across six European countries showed that of 602 patients, 14% had trigeminal neuralgia Citation[13]. They went on to show that the mean age of these 82 patients with trigeminal neuralgia was 62 years and that there was a predominance of females (67%) Citation[14]. Interestingly, they also reported on the treatments of these patients, comparing the drugs that the physician reported they used with patient reports: as would be expected, there were discrepancies. Just over half the patients – 55% – were prescribed one or two antiepileptic drugs, of which carbamazepine was used in 54% of cases, gabapentin in 29% and a variety of others in the rest. However, 46% were still using standard analgesic drugs and 26% were using antidepressant drugs. Up to 40% of patients reported that they used paracetamol and 17% used herbs, vitamins and supplements. The majority of the patients had used their drugs for over 3 years. Yet it is well known that carbamazepine is a highly effective drug, with a number needed to treat (NNT), depending on the review one uses, of around 1.9 [95% confidence interval (CI) 1.4 – 2.8] and – especially early in the history – gives not just 50% pain relief but virtually 100%. In the UK, carbamazepine is the drug of first choice of the British National Formulary and in guidance to GPs in their Clinical Knowledge Summaries. This therefore begs the question, are these patients misdiagnosed or are they being offered suboptimal care due to ignorance of healthcare practitioners? European studies have shown that, generally, chronic pain is vastly undertreated Citation[15].
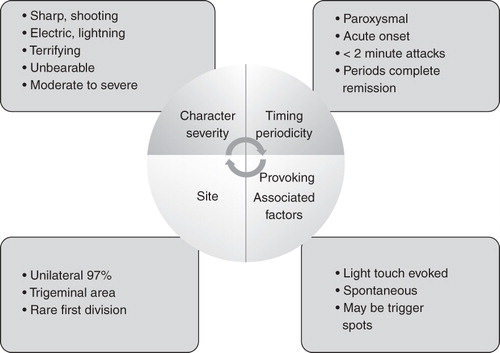
Although it is now recognized that risk factors for trigeminal neuralgia include multiple sclerosis and hypertension, there is still very little data to determine the prognosis for these patients and there have been no comparative studies looking at outcomes of patients who have been medically managed as opposed to those managed surgically. Overall, it appears that relapse is associated with increased intensity of pain; however, although this tends to occur, there are groups of patients in whom the intensity of the pain seems to remain fairly steady. There are few data to determine whether tolerance to drugs develops. The diagnostic clinical features of trigeminal neuralgia are illustrated in .
Experts working in the field of trigeminal neuralgia recognize a form, often termed ‘atypical trigeminal neuralgia’, in which patients not only have the classic features of trigeminal neuralgia but also report a background burning, dull type of pain that does not appear to be as responsive to anticonvulsant therapy as the classic trigeminal neuralgia Citation[16]; it may in fact have a different aetiology. An accurate history of the pain is extremely important as this can alter drug management.
The aetiology and pathophysiology of trigeminal neuralgia has remained difficult to determine and the most widely accepted theory is that of Devor et al. Citation[17]: the ignition hypothesis. There is now increasing evidence that changes occur not only peripherally but also centrally, and that these central changes might be more frequent in patients who suffer from atypical trigeminal neuralgia Citation[18]. Obermann et al. Citation[18] have shown in a small group of patients that there may be overactivation of the central facilitation of trigeminal nociceptive processing. It has often been suggested that the atypical form follows on from classic trigeminal neuralgia, and yet this counters some recent epidemiological studies based on a patient led survey in over 6000 patients in whom the age of onset of classic trigeminal neuralgia is later than that of atypical trigeminal neuralgia and these atypical features are reported at the start of the condition not just in later stages (Velly et al., unpublished data).
Antiepileptic drugs (AED) have been used since the 1860s and possibly relate to the observation that the intermittency of the attacks of pain were similar to epilepsy and that therefore the mechanisms could be similar and patients would respond to these types of drug. In 1942, phenytoin was first reported as being effective, but this was soon superseded by carbamazepine, the use of which in trigeminal neuralgia was first reported in 1962. Since then, many AEDs, and other drugs, have been used for the management of trigeminal neuralgia. There have now been a variety of systematic reviews, both within the Cochrane collaboration and without, to evaluate the use of these drugs Citation[19-23]. These have led to the publication of international guidelines Citation[24,25].
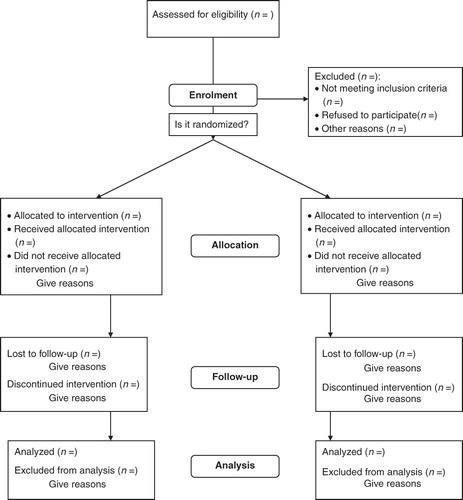
Given the rarity of the condition and its peculiar feature of unpredictable remission periods, it has been difficult to perform robust randomized controlled trials (RCTs). Reviews have shown that many have flawed methodology and do not follow the CONSORT (Consolidated Standards of Reporting Trials; http://www.consort-statement.org) guidelines for reporting of trials ().
Figure 2. The CONSORT flowchart (www.consort-statement.org).
Jorns and Zakrzewska Citation[26] have highlighted these difficulties and have put forward some suggestions for more robust designs of such trials:
Careful phenotyping of patients, paying particular attention to the character of pain, duration of disease and past drugs and responses.
Use of enhancement-enriched studies rather than the conventional cross-over and parallel studies.
Clear statements on primary and secondary outcome measures.
Use of power calculations.
Only very recent published trials follow these recommendations and most outcome measures are based purely on a visual analogue scale of pain intensity. The IMMACT group Citation[27] has suggested a wider range of outcome measures, including quality of life, physical functioning and emotional functioning; the all-important tolerability and safety should also be reported. Currently, only one trial on trigeminal neuralgia, using IV immunoglobulin is listed in the UK Clinical Trials Register. The website http://public.ukcrn.org.uk, lists trials that fulfil the criteria for RCTs and have ethical approval.
2.2 Methods
The author regularly updates a review in Clinical Evidence. The editorial team developed the search strategy, runs the searches and ensures that authors critically appraise all the identified studies using their methodology for quality scoring Citation[23]. Currently, searches have been carried on an update of a Cochrane review on non-epileptic drugs in trigeminal neuralgia Citation[22] and this has also been used to identify all the high-quality studies.
RCTs vary in size from 341 patients to just three patients. lists the drugs used in RCTs with more than 10 patients.
Table 1. Drugs used in trigeminal neuralgia which have been evaluated in randomized controlled trials (RCTs).
2.2.1 Baclofen
Both the racemic and the l forms have been reported in three studies used either on their own or in a head-to-head comparison with carbamazepine Citation[28-30]. All three studies are of poor quality, with small numbers, lasting no more than 2 weeks with no details on randomization; up to 30% withdrew from the Parekh et al. Citation[29] study so the results must be interpreted with care.
2.2.2 Carbamazepine
Three cross-over RCTs comparing carbamazepine with placebo Citation[31-33], involving in total 161 patients, were small, short term, used poor pain outcome measures with no quality-of-life measures and provided few characteristics of patients enrolled or of how allocation concealment was achieved. Although an RCT, the study by Rockliff and Davies Citation[34] had only had nine participants. Killian and Fromm Citation[32] and Nicol Citation[33] extended their studies after 2 weeks up to 46 months. Both studies show evidence of high efficacy. Other studies have been head-to-head comparisons with other drugs baclofen, oxcarbazepine, tizanidine and will be reported below.
Carbamazepine Retard is useful as a night dose to ensure that levels do not fall; up to two-thirds of patients will have no pain at night Citation[35]. Miller et al. Citation[36], however, also suggest that it might decrease side effects as high serum peaks are not achieved and this tends to be when patients experience side effects.
The long-term efficacy of carbamazepine has been reported in only one study Citation[37]. This retrospective study assessed 143 patients for up to 16 years and showed diminished efficacy but this be due to increasing severity of pain.
2.2.3 Gabapentin
This AED has been used extensively in RCTs of neuropathic pain and is proven to be of value. It is therefore not surprising that it has been reported in trigeminal neuralgia in several open studies Citation[38,39] and reviewed Citation[40,41]. Only one RCT used it in combination with ropivacain Citation[42]. Although considerable effort was placed on allocation concealment and randomization, the study was only single blinded as patients knew whether they received an injection. The study was extended beyond 28 days for up to 6 months. The combination patients had the best outcome and were able to use lower total daily doses of gabapentin. The doses used were low – up to 900 mg daily – in comparison with open-label studies, in which up to 3600 mg were used. Although quality of life was reported, there are no details of adverse events, with just a mention in the discussion that the combined treatment group had none.
2.2.4 Lamotrigine
This drug has been reported in small case series Citation[43,44] but has been used in only one small RCT, where it was added to existing sub-effective therapy Citation[45]. Although the NNT has been calculated at 2.1 and the 95% confidence limits are wide (1.3 – 6.1), its effectiveness as a stand-alone drug has not been fully evaluated. It is essential to escalate the dose very slowly.
2.2.5 Oxcarbazepine
The keto derivative of carbamazepine does not pass through the liver cytochrome system and so results in fewer side effects and drug interactions than carbamazepine. Unfortunately, the RCTs and meta- analysis Citation[46-48] remain as abstracts and it is impossible to get further information to evaluate these trials comprehensively. They compared carbamazepine to oxcarbazepine and showed equal efficacy: 88% received a pain reduction > 50% but tolerability was better with oxcarbazepine. Three case reports Citation[49-51] show similar efficacy. A long-term cohort study on patients with oxcarbazepine show that with time the disease becomes more severe and surgery becomes the treatment of choice Citation[52].
2.2.6 Other small randomized controlled studies
Dextromethorphan Citation[53] and topiramate Citation[54] were both evaluated in an RCT involving three patients, which is too small to provide any meaningful data. Eye drops Citation[55] and streptomycin injections with lidocaine Citation[56], when assessed in RCTs, showed no effect. Tocainade Citation[57] was compared with carbamazepine in a small study and was shown to have the same effectiveness but more adverse effects. Another two small RCTs with tizanidine showed this drug to have results similar to carbamazepine Citation[58,59].
A recent novel approach is subcutaneous sumatriptan Citation[60]. The premise was that there might be a role for a 5-HT receptor in trigeminal neuralgia. Patients had to come off all their neuralgic medications for 12 h prior to the trial commencing and the outcome measure was whether the pain could be triggered 15 min after the injection. The effect persisted for only 8 h. The authors suggest that the intranasal spray could be used to control severe evoked attacks of pain.
2.3 Drugs used in case series trigeminal neuralgia
Most AEDs have been used and reported in case series for trigeminal neuralgia, including phentyoin Citation[61-63], valproic acid Citation[64], clonazepam Citation[65-68] and felbamate Citation[69] (). More robust case studies have showed that leviteracetam, although having very good tolerability, is not effective in trigeminal neuralgia Citation[70].
Table 2. Drugs used in case series only for trigeminal neuralgia.
A well-designed cohort study using pregablin in doses of 150 – 600 mg daily in 53 patients over a period of 1 year shows promise. Pregablin has been found to be particularly useful in those patients who did not report other concomitant facial pain Citation[71]. A study in primary care by a Spanish group showed that pregablin was cost effective as its effectiveness reduced other healthcare costs Citation[72]. The side effects profile was similar to other AEDs but was less marked, i.e., dizziness, tiredness, headache and peripheral oedema. Its advantage over other AEDs is that it can be used on a twice-daily dosage scheme and can be titrated rapidly. Both studies showed that the results were not sustained and other AEDs were needed later.
Topical capsaicin has also been reported Citation[73,74] but it is painful to put on especially if the pain is triggered by touch.
Although trigeminal neuralgia is considered to be due to neurovascular compression, a small, open-label study using human pooled immunoglobulin in chronic pain patients showed that, of the groups responding, the best results were seen in patients with trigeminal neuralgia, with four out of six patients having more than 70% pain relief Citation[75]. This is now going to be tested in an RCT that has been set up and is on the clinical trials register Citation[76].
A report of three cases suggests that an infusion of fosphenytoin might be useful in acute cases Citation[69].
Patients with trigeminal neuralgia and multiple sclerosis are difficult to treat and do not respond well to conventional AEDs. On the basis of 11 patients, Khan Citation[77] and Solaro et al. Citation[78] propose either gabapentin on its own or the combination of low-dose gabapentin with either lamotrigine or carbamazepine. The DMKG group Citation[79] evaluated misoprostol in 14 patients and showed benefit in the short term. Topiramate was found to be effective in six patients Citation[80].
3. Glossopharyngeal neuralgia
3.1 Overview
The International Association for the Study of Pain defines glossopharyngeal neuralgia as a sudden severe brief recurrent pain in the distribution of the glossopharyngeal nerve Citation[8]. In most cases, this condition is idiopathic but some instances might be due to symptomatic causes, again compression of the nerve by tumours or malformations. Glossopharyngeal pain is also a severe, transient, sharp, stabbing pain but its distribution is along the route of the glossopharyngeal nerve and so it is felt in the ear, at the base of the tongue, at the tonsillar fossa and beneath the angle of the jaw. The pain can also be felt in the auricular and pharyngeal branches of the vagus nerve. It is provoked by light touch activities such as swallowing, talking or coughing. Patients will suffer from episodes of pain lasting for weeks or months, and then have periods of remission. The attacks themselves also last for no more than 2 min. The pain again is unilateral.
Glossopharyngeal neuralgia is even rarer, with an incidence rate of 0.7 per 100,000, and it has been reported as coexisting with trigeminal neuralgia Citation[81]. It occurs in older age groups and seems to predominate in women. There are no data on prognosis but, judging by the few reports of surgical treatment, it would appear that patients have a less severe history than those with trigeminal neuralgia. There are no trials reporting the use of any drugs in glossopharyngeal neuralgia. The largest review of patients with trigeminal neuralgia, by Rushton Citation[82], suggested the same drugs as for trigeminal neuralgia and half of the patients eventually underwent surgical management. Other drugs have been reported mainly as single-case reports: pregabalin Citation[83], lamotrigine Citation[84], oxcarbazepine Citation[85], carbamazepine Citation[86], gabapentin Citation[87].
4. Traumatically induced neuralgia, trigeminal neuropathic pain and atypical odontalgia
4.1 Overview
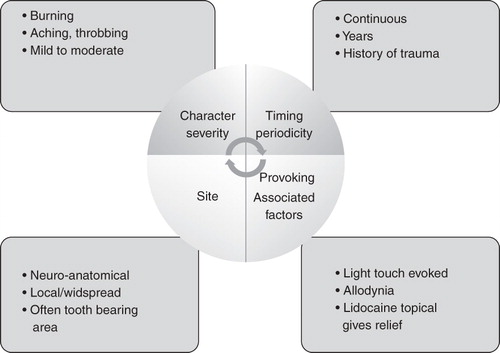
It is now increasingly recognized that trauma to the trigeminal nerve can result not just in neuropathy but also long-term neuropathic pain. Many cases previously diagnosed as atypical facial pain are probably cases that in fact belong to this group. Careful neurophysiological and qualitative physiological testing can distinguish these conditions, which could be of importance in management Citation[88]. There is also the highly specific condition ‘atypical odontalgia’, which is defined as pain in a tooth, or a tooth-bearing area, which is not related to any dental cause and again is often mistaken as toothache and treated with multiple dental treatments Citation[12]. These pains may in fact constitute a sub-set of trigeminal neuropathic pain and have been well characterized by Baad-Hansen Citation[89] and List et al. Citation[90]. Neurophysiological testing shows that these patients have peripheral and central sensitization changes but there is also some evidence for nociceptive changes, which might therefore be important in the choice of drugs Citation[91] Currently, there are no data on the epidemiology of neuropathic pain but it has been suggested that a risk factor for this could be inadequate anaesthesia during dental procedures, as this increases the risk for potential central sensitization. As anywhere else in the body, trauma and compression of sensory nerves can result in long term neuropathic pain.
The clinical features of trigeminal neuropathic pain are shown in the .
4.2 Management
In a review of clinical management of atypical odontalgia, Baad-Hansen Citation[89] suggests that most of the management is based on expert opinion and case reports. Most experts recommend the use of tricyclic antidepressants but the anticonvulsants, such as phenytoin, carbamazepine, pregabalin and gabapentin, have also been proposed. Topical analgesics such as lidocaine or capsaicin have been put forward as first-line treatments due to their lack of systemic side-effects. There has only been one specific randomized control trial of management of atypical odontalgia. List et al. Citation[91] performed a randomized cross-over study in 35 patients with atypical odontalgia, which involved either injection of lidocaine with adrenalin or normal saline. Those receiving the local anaesthetic were more likely to achieve pain relief but this was not complete, suggesting that the pain of atypical odontalgia is not purely dependent on peripheral afferent inputs and that there may be more central sensitization. This would potentially indicate that use of topical lidocaine in the form of patches or gels would be insufficient to give complete pain relief.
There has been a move to tailor the treatment of neuropathic pain more to the mechanisms involved and so to target specific areas, such as sodium and calcium channels, glutamate receptors, monoamines and neurotrophic factors. However, as yet there is insufficient detail on many of these pain mechanisms to be able to put them into categories. There is increasing evidence that in many cases there is some overlap between neuropathic pain and nociceptive pain and hence drugs such as the opiods may be useful as add on medications. Treatment of trigeminal neuropathic pain is based on extrapolation from the data on neuropathic pain using a variety of the different guidelines that are now in place, these are summarized in .
Table 3. Summary of drugs used in neuropathic pain based on a review of the O'Conner and Dworkin guidelines, which could be used in trigeminal neuropathic pain.
O'Connor and Dworkin Citation[92] have recently reviewed all the guidelines on neuropathic pain, including the European Federation of Neurological Society guidelines EFNS (Attal et al. Citation[93]), the Canadian guidelines Citation[94] and those produced by the Neuropathic Special Interest Group NeuPSIG Citation[95]. They note that the major differences relate to those areas in which there is a lack of evidence. There is consensus in all three guidelines that tricyclic antipressants and calcium channel blockers (gabapentin and pregablin) should be first-line drugs. The SSRIs/SSNRIs duloxetine and venalafaxine are also considered first line by the NeuSig guidelines, but second line by the Canadian and EFN guidelines. Topical lidocaine is advocated as first line in peripheral neuropathic pain by the NeuPSIG and EFNS guidelines. Opioids and tramdadol are all second or even third line in the Canadian ones. Despite this reasonable body of evidence, patients in primary care continue to be poorly managed and opioids are used much more frequently than the guidelines would suggest Citation[96].
There is a need for individualization of medication based on other co-morbidities and the use of other medications. Attention is drawn to the fact that there is a lack of long-term cohort studies and head-to-head comparisons between different medications and combinations of medications. Trials also suggest that higher doses do not always result in improved efficacy but do result in increased side effects. The majority of the drugs need to be slowly escalated and used for several weeks before they are deemed ineffective.
5. Burning mouth syndrome
5.1 Overview
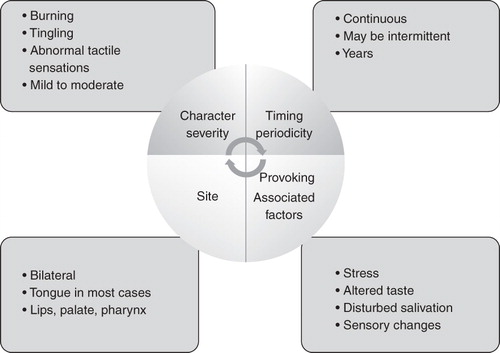
Burning mouth syndrome (BMS), or stomatodynia, is defined as a chronic, idiopathic oral mucosal pain/discomfort in which no clinical lesions or systemic diseases are identified. Its principal features are indicated in .
It is important to exclude other causes – both local and systemic – that can cause burning especially drugs, such as the ACE inhibitors. It is particularly common in peri-menpausal women who are often told that this is due to psychological cause. However, there is now an increasing body of evidence to show that there are neuropathic elements to this pain Citation[97] and there are several RCTs and systematic reviews on this topic:
World workshop in oral medicine Citation[98].
Clinical Evidence (www.clinicalevidence.bmj.com) Citation[99].
Cochrane systematic review on this topic Citation[100].
The whole topic has been well covered by Woda and Grushka in a recent chapter on this topic Citation[97]. The treatments used in BMS are summarized in .
Table 4. Drugs used in burning mouth syndrome based on table in ref.Citation[97].
Of the RCTs, it would appear that clonazepam used topically may be helpful as well as cognitive behaviour therapy. Many patients who are given a physiological explanation for their pain, and reassurance that they are not developing cancer, learn to cope with their symptoms and do not require drug therapy.
6. Conclusion
Several neuropathic pain conditions occur in the face and mouth, the majority of which are treated like other neuropathic pains using drugs such as tricyclic antidepressants, gabapentin and pregablin. However, patients with trigeminal neuralgia respond best to AEDs such as carbamazepine and oxcarbazepine.
7. Expert opinion
A major problem encountered by all practising clinicians is trying to extrapolate the findings from RCTs, as many patients have other co-morbities and psychosocial problems that can have a profound effect on their response to drug therapy. It is important to ensure that the drugs are used in the dosages that have been used in the trials as often patients are given suboptimal dosages and so the drugs are perceived to be ineffective.
7.1 Trigeminal neuralgia
There is little doubt as to the efficacy of carbamazepine in the management of trigeminal neuralgia. The drug is highly effective, provides excellent pain relief within a few days, especially initially, but its widespread side effects and drug interactions make it a complex drug to use in clinical practise.
Oxcarbazepine, therefore, is a very useful alternative as it has similar efficacy to carbamazepine but greatly improved tolerability and relative lack of interaction with other drugs. Having used both drugs for over 20 years in large cohorts of studies, I am in no doubt that oxcarbazepine is better tolerated and patients particularly find the reduction in cognitive impairment to improve their quality of life.
Lamotrigine is a useful drug in patients who develop allergies to carbamazepine and oxcarbazepine, but the need for slow escalation to reduce skin reactions means it is not useful for acute cases. It might also be useful in those patients who have been mistakenly diagnosed as trigeminal neuralgia but in fact have SUNCT or SUNA.
Although I have used gabapentin as a third-line drug, I have had disappointing results with it and have had to use much higher dosages than those reported in the randomized controlled trial Citation[42]. Although the side effects of gabapentin are quoted as being low, weight gain and oedema seem to be more frequently reported than when using other anticonvulsants.
Pregabalin appears to be a promising drug in that it can be escalated more rapidly than gabapentin, can be used on a twice daily dosage scheme and appears to have some effect on reducing anxiety often present in these patients. I find it of more value in patients with trigeminal neuropathic pain than trigeminal neuralgia.
There are very few data to support the use of polypharmacy but if patients with trigeminal neuralgia report that they also have a dull, burning, aching background pain then I often will include a tricyclic antidepressant such as nortriptyline in doses around 40 mg nocte. Once patients have gained confidence in managing their drug regimen, they are very reluctant to change to other drugs as they fear that a changeover could escalate their symptoms, which is a major cause for lack of recruitment to our IVG study Citation[76]. In our open trial of leviteracetam Citation[70] there was a very marked improvement in side effects, even when used in high doses, but the drug was disappointingly non-effective in severe cases of trigeminal neuralgia.
Any drug that was to replace carbamazepine as the gold standard for the treatment of trigeminal neuralgia would need to have the following minimal characteristics:
1) As effective in terms of pain relief.
2) Improved therapeutic index (i.e., less toxic in relation to its observed benefits).
3) Simple pharmacokinetic characteristics both in relation to the prescribing of the drug and patient compliance. Of special importance would be the ability to escalate the drug rapidly.
4) Should not interact with other drugs which may be co-prescribed as many of these patients are elderly and have other co-morbidities.
7.2 Glossopharyngeal neuralgia
Glossopharyngeal neuralgia is best managed with the use of carbamazepine, but quickly changing to oxcarbazepine if there are any issues surrounding side effects and drug interactions. I have not had any experience in using any of the other drugs as I have found one or other of these drugs to be effective in my dozen or so cases.
7.3 Trigeminal neuropathic pain
The increasing recognition of this condition puts increasing challenges on clinicians to manage this pain. A topical approach is the use of lidocaine or capsaicin patches, or even clonazepam, as used in burning mouth syndrome. It may provide some benefit, especially if the pain is provoked by light touch activities and interferes with sleep. Some patients have found that the advantage of having a good night's sleep enables them to cope better with their neuropathic pain throughout the day. However, as the trials have shown, it is highly likely that trigeminal neuropathic pain also results in central changes and therefore there is a requirement for systematic drugs. Nortriptyline, often in lower doses than recommended in the guidelines, seems to result in a 30% pain reduction. Pregabalin appears to be especially useful in patients who also show high level of anxiety. Topical lidocaine may again be useful in those patients whose sleep is interrupted due to the allodynia.
7.4 Patient education
Patient education is crucial, the more patients who understand their condition and how the drugs work for them, the more effective the drugs become. Patients are keen to have this information, as seen by the size of their support groups (www.tna.org.uk, www.fpa-support.org) and a general willingness to publish books on this topic (e.g., Insights – facts and stories behind trigeminal neuralgia Citation[101]). Patients are often referred to me as medical failures but it is often as much the physician's fault as the patient's that the pain is not being controlled. Many patients with BMS, when reassured and given an explanation, will manage without drug therapy. Patients with trigeminal neuralgia need to take their medication before meal times or washing, as these are the prime times when their pain is evoked, and yet many have the belief that drugs should be taken after meals to reduce side effects. It needs to be made clear which drugs need to be taken on a frequent dosage scheme and which can be taken twice daily. The keeping of diaries for short periods is useful if a patient is to gain an understanding of his or her individual response to the drugs and then to be able to take control. This is especially important in patients with intermittent pain, such as trigeminal neuralgia, as they need to be able to both escalate and decrease their drugs dependent on the periodicity of the pain.
7.5 Future directions
Katz et al. Citation[102] looked at clinical trial outcomes in neuropathic pain to identify factors leading to positive or negative outcomes. This study again provides impetus for future research, suggesting the need for decreasing placebo response rates, looking at different trial designs and at enrolment of larger numbers of subjects. Straube et al. Citation[103] reviewed the value of enhancement enrichment enrolment studies in potential trials of pregabalin and gabapentin in neuropathic pain. These types of study enable a pilot study to be carried out initially and those patients who respond to the drug therapy are then enrolled into a randomized controlled trial. The outcome measure is the speed with which patients drop out of the study, which in theory should be faster for those given the placebo. They suggest that no great benefit was found in current studies but that, if future researchers are to use this methodology then any enrichment must describe both the process and the extent of enrichment; only time will tell whether this methodology may be useful.
There is a need for head-to-head comparisons of the range of drugs available; it would seem from data available that newer drugs appear to provide the same pain relief profile but are associated with fewer side effects. Mechanism-based treatments are probably the way forward as we increasingly recognize that neuropathic pain is multifactorial and more than one mechanism might be involved. Thus, a combination of drugs that act on different sites might be needed. Future trials might need to consider the addition of second-line drugs or opioids once maximum relief has been obtained with first-line drugs. There is also some evidence that there may be an immunological response to neuropathic pain, as early work by Goebel et al. Citation[75] suggested, and the ongoing RCT of intravenous immunoglobulin in trigeminal neuralgia may show other mechanisms to be involved, especially in those patients who are intractable and do not have neurocompression.
The findings from trials need to be rapidly communicated to primary care as numerous studies have shown how inadequately chronic pain patients are managed Citation[15,14,96] and patient education needs to be high on the agenda.
Let us bear in mind that our future research needs to be patient centred and that, at present, our patients continue to struggle on the drugs we prescribe. This is aptly expressed by Betty Price, a patient with trigeminal neuralgia who writes Citation[101]:
“There is a medicine you can take
But it's not the cure
It may make you drowsy
It may make you sick
Now he was not kidding
For this it quickly did
There are more pills to take
And there's no miracle fix
Well days have come and gone
The nausea is still the same
I feel I'm fighting a battle
With little coasting along the way.”
Article highlights.
There are few high quality RCTs of drugs used all these conditions.
Carbamazepine and oxcarbazepine are the first line drugs for trigeminal neuralgia and glossopharyngeal neuralgia.
Trigeminal neuropathic pain also called atypical odontalgia is managed using neuropathic type drugs.
Burning syndrome is likely to be a neuropathic pain.
Management begins with education and first line drug is topical clonazepam followed by other neuropathic type drugs.
Declaration of interest
J. Zakrzewska has received investigator led grant from UCB Pharma for study on leviteracetam. This work was undertaken at UCL/UCLHT who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centre funding scheme.
Notes
This box summarises key points contained in the article.
Bibliography
- Cohen AS, Matharu MS, Goadsby PJ. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA) – a prospective clinical study of SUNCT and SUNA. Brain 2006;129:2746-6
- Anonymous. The international classification of headache disorders: 2nd edition. Cephalalgia 2004;24(Suppl 1):9-160
- Zakrzewska JM. Diagnosis and differential diagnosis of trigeminal neuralgia. Clin J Pain 2002;18:14-21
- Woolf CJ, Bennett GJ, Doherty M, Towards a mechanism-based classification of pain? Pain 1998;77:227-9
- Finnerup NB, Sindrup SH, Jensen TS. Chronic neuropathic pain: mechanisms, drug targets and measurement. Fundam Clin Pharmacol 2007;21:129-36
- Bennett MI, Attal N, Backonja MM, Using screening tools to identify neuropathic pain. Pain 2007;127:199-203
- Fisher A, Zakrzewska JM, Patsalos PN. Trigeminal neuralgia: current treatments and future developments. Expert Opin Emerg Drugs 2003;8:123-43
- Merskey H, Bogduk N. Classification of chronic pain. Descriptors of chronic pain syndromes and definitions of pain terms., 2nd Edition. IASP Press, Seattle, 1994
- Zakrzewska JM, Hamlyn PJ. Facial pain. In: Crombie IKCPR, Linton SJ, LeResche L, Von Korff M, editors, Epidemiology of pain, 1st Edition. IASP Press, Seattle, 1999
- Dieleman JP, Kerklaan J, Huygen FJ, Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008;137:681-8
- Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain 2006;122:156-62
- Drangsholt M, Truelove E. Trigeminal neuralgia mistaken as temporomandibular disorder. J Evid Based Dent Pract 2001;1:41-50
- McDermott AM, Toelle TR, Rowbotham DJ, The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 2006;10:127-35
- Tolle T, Dukes E, Sadosky A. Patient burden of trigeminal neuralgia: results from a Cross-Sectional Survey of health state impairment and treatment patterns in six European Countries. Pain Pract 2006;6:153-60
- Breivik H, Collett B, Ventafridda V, Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333
- Limonadi FM, McCartney S, Burchiel KJ. Design of an artificial neural network for diagnosis of facial pain syndromes. Stereotact Funct Neurosurg 2006;84:212-20
- Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 2002;18:4-13
- Obermann M, Yoon MS, Ese D, Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 2007;69:835-41
- Wiffen PJ, McQuay HJ, Moore RA. Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev 2005;3:CD005451
- Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev 2005;3:CD005452
- Wiffen PJ, Rees J. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev 2007;2:CD006044
- He L, Wu B, Zhou M. Non-antiepileptic drugs for trigeminal neuralgia. Cochrane Database Syst Rev 2006;3:CD004029
- Zakrzewska JM, Linskey ME. Trigeminal neuralgia. Clin Evid 2009;1207
- Cruccu G, Gronseth G, Alksne J, AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol 2008;15:1013-28
- Gronseth G, Cruccu G, Alksne J, Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology 2008;71:1183-90
- Jorns TP, Zakrzewska JM. Evidence-based approach to the medical management of trigeminal neuralgia. Br J Neurosurg 2007;21:253-61
- Dworkin RH, Turk DC, Farrar JT, Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19
- Fromm GH, Terrence CF, Chattha AS. Baclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-up. Ann Neurol 1984;15:240-4
- Parekh S, Shah K, Kotdawalla H, Gandhi I. Baclofen in carbamazepine resistant trigeminal neuralgia – a double blind clinical trial. Cephalalgia 1989;9:392-3
- Fromm GH, Terrence CF. Comparison of L-baclofen and racemic baclofen in trigeminal neuralgia. Neurology 1987;37:1725-8
- Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbamazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry 1966;29:265-7
- Killian JM, Fromm GH. Carbamazepine in the treatment of neuralgia. Arch Neurol 1968;19:129-36
- Nicol CF. A four year double-blind study of tegretol in facial pain. Headache 1969;9:54-7
- Rockliff BW, Davis EH. Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol 1966;15:129-36
- Devor M, Wood I, Sharav Y, Zakrzewska JM. Trigeminal neuralgia during sleep. Pain Pract 2008
- Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand 2004;109:374-7
- Taylor JC, Brauer S, Espir MLE. Long-term treatment of trigeminal neuralgia. Postgrad Med J 1981;57:16-8
- Sist T, Filadora V, Miner M, Lema M. Gabapentin for idiopathic trigeminal neuralgia: report of two cases. Neurology 1997;48:1467
- Valzania F, Strafella AP, Nassetti SATA, Tassinari CA. Gabapentin in idiopathic trigeminal neuralgia. Neurology 1998;50(4):A379
- Cheshire WP Jr. Defining the role for gabapentin in the treatment of trigeminal neuralgia: a retrospective study. J Pain 2002;3:137-42
- Serpell MG. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain 2002;99:557-66
- Lemos L, Flores S, Oliveira P, Almeida A. Gabapentin supplemented with ropivacain block of trigger points improves pain control and quality of life in trigeminal neuralgia patients when compared with gabapentin alone. Clin J Pain 2008;24:64-75
- Lunardi G, Leandri M, Albano C, Clinical effectiveness of lamotrigine and plasma levels in essential and symptomatic trigeminal neuralgia. Neurology 1997;48:1714-17
- Canavero S, Bonicalzi V, Ferroli P, Lamotrigine control of idiopathic trigeminal neuralgia [letter]. J Neurol Neurosurg Psychiatry 1995;59:646
- Zakrzewska JM, Chaudhry Z, Nurmikko TJ, Lamotrigine (lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain 1997;73:223-30
- Liebel JT, Menger N, Langohr H. Oxcarbazepine in der Behandlung der Trigeminusneuralgie. Nervenheilkunde 2001;20:461-5
- Beydoun A, Kutluay E. Oxcarbazepine. Expert Opin Pharmacother 2002;3:59-71
- Beydoun A, Schmidt D, D'souza J. Oxcarbazepine versus carbamazepine in trigeminal neuralgia : a meta-anlaysis of three double blind comparative trials. Neurology 2002;S3:2
- Zakrzewska JM, Patsalos PN. Oxcarbazepine: a new drug in the management of intractable trigeminal neuralgia. J Neurol Neurosurg Psychiatry 1989;52:472-6
- Farago F. Trigeminal neuralgia: its treatment with two new carbamazepine analogues. Eur Neurol 1987;26:73-83
- Remillard G. Oxcarbazepine and intractable trigeminal neuralgia. Epilepsia 1994;35:s28-9
- Zakrzewska JM, Patsalos PN. Long-term cohort study comparing medical (oxcarbazepine) and surgical management of intractable trigeminal neuralgia. Pain 2002;95:259-66
- Gilron I, Booher SL, Rowan MS, A randomized, controlled trial of high-dose dextromethorphan in facial neuralgias. Neurology 2000;55:964-71
- Gilron I, Booher SL, Rowan JS, Max MB. Topiramate in trigeminal neuralgia: a randomized, placebo-controlled multiple crossover pilot study. Clin Neuropharmacol 2001;24:109-12
- Kondziolka D, Lemley T, Kestle JR, The effect of single-application topical ophthalmic anesthesia in patients with trigeminal neuralgia. A randomized double-blind placebo-controlled trial. J Neurosurg 1994;80:993-7
- Bittar GT, Graff-Radford SB. The effects of streptomycin/lidocaine block on trigeminal neuralgia: a double blind crossover placebo controlled study. Headache 1993;33:155-60
- Lindstrom P, Lindblom V. The analgesic effect of tocainide in trigeminal neuralgia. Pain 1987;28:45-50
- Fromm GH, Aumentado D, Terrence CF. A clinical and experimental investigation of the effects of tizanidine in trigeminal neuralgia. Pain 1993;53:265-71
- Vilming ST, Lyberg T, Latase X. Tizanidine in the management of trigeminal neuralgia. Cephalalgia 1986;6:181-2
- Kanai A, Saito M, Hoka S. Subcutaneous sumatriptan for refractory trigeminal neuralgia. Headache J Head Face Pain 2006;46:577-82
- Iannone A, Baker AB, Morrell F. Dilantin in the treatment of trigeminal neuralgia. Neurology 1958;8:126-8
- Braham J, Saia A. Phenytoin in the treatment of trigeminal and other neuralgias. Lancet 1960;2:892-3
- Chinitz A, Seelinger DF, Greenhouse AH. Anticonvulsant therapy in trigeminal neuralgia. Am J Med Sci 1966;252:62-7
- Peiris JB, Perera GL, Devendra SV, Lionel ND. Sodium valproate in trigeminal neuralgia treatment of tic douloureux with a new anticonvulsant (clonazepam). Med J Aust 1980;2:278-9
- Caccia MR. Clonazepam in facial neuralgia and cluster headache. Clinical and electrophysiological study. Eur Neurol 1975;13:560-3
- Chandra B. The use of clonazepam in the treatment of tic douloureux (a preliminary report). Proc Aust Assoc Neurol 1976;13:119-22
- Court JE, Kase CS. Treatment of tic douloureux with a new anticonvulsant (clonazepam). J Neurol Neurosurg Psychiatry 1976;39:297-9
- Smirne S, Scarlato G. Clonazepam in cranial neuralgias. Med J Aust 1977;1:93-4
- Cheshire WP. Felbamate relieved trigeminal neuralgia. Clin J Pain 1995;11:139-42
- Jorns TP, Johnston A, Zakrzewska JM. Pilot study to evaluate the efficacy and tolerability of leviteracetam (keppra) in the treatment of patients with trigemional neuralgia. Eur J Neurol 2009;16:740-4
- Obermann M, Yoon MS, Sensen K, Efficacy of pregabalin in the treatment of trigeminal neuralgia. Cephalalgia 2008;28:174-81
- Perez C, Navarro A, Saldana MT, Patient-reported outcomes in subjects with painful trigeminal neuralgia receiving pregabalin: evidence from medical practice in primary care settings. Cephalalgia 2009;29:781-90
- Fusco BM, Alessandri M. Analgesic effect of capsaicin in idiopathic trigeminal neuralgia. Anesth Analg 1992;74:375-7
- Epstein JB, Marcoe JH. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol 1994;77:135-40
- Goebel A, Netal S, Schedel R, Sprotte G. Human pooled immunoglobulin in the treatment of chronic pain syndromes. Pain Med 2002;3:119-27
- Goebel A, Moore A, Weatherall R, Intravenous immunoglobulin in the treatment of primary trigeminal neuralgia refractory to carbamazepine: a study protocol [ISRCTN33042138]. BMC Neurol 2003;3:1
- Khan OA. Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology 1998;51:611-14
- Solaro C, Messmer UM, Uccelli A, Low-dose gabapentin combined with either lamotrigine or carbamazepine can be useful therapies for trigeminal neuralgia in multiple sclerosis. Eur Neurol 2000;44:45-8
- DMKG study group. Misoprostol in the treatment of trigeminal neuralgia associated with multiple sclerosis. J Neurol 2003;250:542-5
- Zvartau-Hind M, Din MU, Gilani A, Topiramate relieves refractory trigeminal neuralgia in MS patients. Neurology 2000;55:1587-8
- Katusic S, Williams DB, Beard CM, Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945-1984. Neuroepidemiology 1991;10:276-81
- Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: a study of 217 cases. Arch Neurol 1981;38:201-5
- Kitchener JM. Glossopharyngeal neuralgia responding to pregabalin. Headache 2006;46:1307-8
- Titlic M, Jukic I, Tonkic A, Use of lamotrigine in glossopharyngeal neuralgia: a case report. Headache 2006;46:167-9
- Luef G, Poewe W. Oxcarbazepine in glossopharyngeal neuralgia: clinical response and effect on serum lipids. Neurology 2004;63:2447-8
- Ekbom KA, Westerberg CE. Carbamazepine in glossopharyngeal neuralgia. Arch Neurol 1966;14:595-6
- Moretti R, Torre P, Antonello RM, Gabapentin treatment of glossopharyngeal neuralgia: a follow-up of four years of a single case. Eur J Pain 2002;6:403-7
- Forssell H, Tenovuo O, Silvoniemi P, Jaaskelainen SK. Differences and similarities between atypical facial pain and trigeminal neuropathic pain. Neurology 2007;69:1451-9
- Baad-Hansen L. Atypical odontalgia – pathophysiology and clinical management. J Oral Rehabil 2008;35:1-11
- List T, Leijon G, Helkimo M, Clinical findings and psychosocial factors in patients with atypical odontalgia: a case-control study. J Orofac Pain 2007;21:89-98
- List T, Leijon G, Helkimo M, Effect of local anesthesia on atypical odontalgia – a randomized controlled trial. Pain 2006;122:306-14
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 2009;122:S22-32
- Attal N, Cruccu G, Haanpaa M, EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 2006;13:1153-69
- Moulin DE, Clark AJ, Gilron I, Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag 2007;12:13-21
- Dworkin RH, O'Connor AB, Backonja M, Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237-51
- Torrance N, Smith BH, Watson MC, Bennett MI. Medication and treatment use in primary care patients with chronic pain of predominantly neuropathic origin. Fam Pract 2007;24:481-5
- Woda A, Grushka M. Burning mouth syndrome. In: Zakrzewska JM, editors, Orofacial Pain. 1st Edition. Oxford University Press, Oxford; 2009
- Patton LL, Siegel MA, Benoliel R, De Laat A. Management of burning mouth syndrome: systematic review and management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(Suppl):S39-13
- Buchanan J, Zakrzewska J. Burning mouth syndrome. Clin Evid 2005;1685-90
- Zakrzewska JM, Forssell H, Glenny AM. Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev 2005;1:CD002779
- Zakrzewska JM. Insights : facts and stories behind trigeminal neuralgia. Gainesville: Trigeminal neuralgia association, 2006
- Katz J, Finnerup NB, Dworkin RH. Clinical trial outcome in neuropathic pain. Relationship to study characteristics. Neurology 2008;70:250-1
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. Br J Clin Pharmacol 2008;66:266-75